Table 2. Scope of heteroarene alkylation and reduction a .
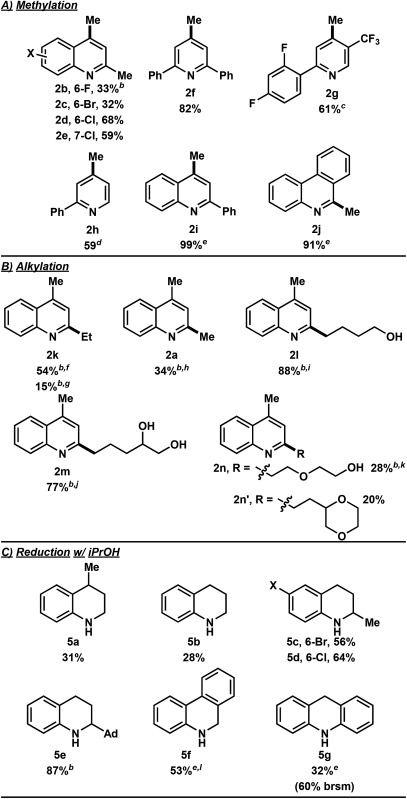
|
aProcedure: 1 (0.4 mmol, 1 equiv.), ROH (0.8 mL), HCl (conc. in H2O, 2.0 mmol, 150 μL), c = 0.42 M, Ar degas, irradiation with 2× UVA LEDs for 8 h. Isolated yields are reported.
b16 h.
c40 h.
d24 h, 20% SM along with 8% 2-methyl-6-phenylpyridine and 6% 2,4-dimethyl-6-phenylpyridine observed.
e410 nm LED was used for 16 h.
fROH = ethanol.
gEt2O used instead of EtOH.
hMTBE used instead of MeOH.
iTHF was used.
jTetrahydrofurfuryl alcohol was used.
k1,4-Dioxane used, 2k also isolated in 26% yield.
