Table 1. . Calcium calmodulin serine/threonine kinase IIδ inhibitors in cardiac muscle.
| No. | Structure | Inhibitor class | Study year | Activity IC50 |
|---|---|---|---|---|
| 1 | Natural | 2012 | 112 µM | |
| 2 | 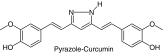 |
Semisynthetic | 2012 | 1.49 µM |
| 3 | 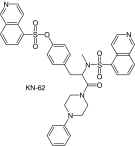 |
Synthetic | 1990 | 500 nM |
| 4 | 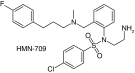 |
Synthetic | 1996 | 1.57 µM |
| 5 |  |
Group of aryl indolyl–maleimide scaffold, synthetic inhibitors | 2008 | 10 nM to >20 µM |
| 6 | 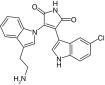 |
Most active aryl indolyl–inhibitor against CaMK IIδ | 2008 | 10 nM |
| 7 | 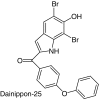 |
Synthetic | 2012 | 12 nM |
| 8 | Homology modeling synthesis of pyrimidine-based inhibitors | 2008 | 0.009–3 µM | |
| 9 | 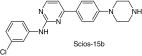 |
Most active pyrimidine-based inhibitor | 2008 | 9 nM |
| 10 | 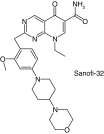 |
Synthetic | 2012 | 2 nM |
| 11 | 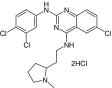 |
Inhibitor resulted from ligand-based virtual screening CaMK IIδ inhibitor | 2011 | 20 nM |
| 12 | 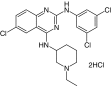 |
Inhibitor resulted from ligand-based virtual screening CaMK IIδ inhibitor | 2011 | 82 nM |
CaMK-IIδ: Calcium calmodulin serine/threonine kinase IIδ.
