Abstract
Background
Level of proximal lymphovascular ligation remains controversial in carcinoma rectum. High-tie inferior mesenteric artery (IMA) claims better lymph node clearance; low-tie IMA minimizes autonomic nerve injury (ANI) and ensures vascularity to anastomosis. Objective of this study is to compare postsurgical complications and oncological clearance in laparoscopic rectal resection (LRR) and open rectal resection (ORR) for carcinoma rectum, with low-tie IMA and selective D3 lymphadenectomy.
Methods
Retrospective analysis was done comparing LRR and ORR done with low-tie IMA for carcinoma rectum/rectosigmoid for significant differences (P<0.05) regarding postsurgical complications and histopathology parameters.
Results
A total of 118 patients; 48 in LRR group and 70 in ORR group were studied. They were comparable in age, site of lesion and clinical TNM (cTNM) stage. Comorbidities and symptoms requiring upfront surgery were more among ORR. 75% LRR and 55.3% ORR had neoadjuvant chemoradiation (NACRT). Duration of surgery was longer in LRR. Clavien-Dindo grade >3 was similar in two groups. Histopathology characteristics were also comparable; including specimen length, lymph node yield, length of distal margin and pathologic TNM (pTNM) stage. Selective D3 lymphadenectomy was done in 37.5% LRR and 37.14% ORR. And 4.16% in LRR and 4.28% in ORR were had positive IMA root lymph nodes.
Conclusions
The post-surgical complications and oncological clearance of LRR done with low-tie IMA and selective D3 lymphadenectomy were found equivalent to ORR. Low-tie IMA without routine splenic flexure mobilisation had no technical issues regarding the anastomosis.
Keywords: Laparoscopic rectal resection (LRR), low-tie IMA, D3 lymphadenectomy, oncological clearance, Clavien-Dindo grade
Introduction
Lymph node involvement is recognised as a major prognostic factor in the survival of resected cases of carcinoma rectum. Extent of lymphadenectomy still remains controversial in rectal cancer surgery; ‘high-tie’ of inferior mesenteric artery (IMA) at its origin from the aorta versus ‘low-tie’ of IMA just distal to the origin of left colic artery (LCA) at the level of the proximal superior rectal artery (SRA).
Miles in 1908 described abdominoperineal resection for rectal cancer; with en bloc lymphadenectomy as low-tie IMA (1). Moynihan proposed high-tie to include IMA root lymph nodes in the resection field (2). High-tie IMA claims better lymph node clearance; but has a higher risk for urogenital dysfunctions because of autonomic nerve injury (ANI) (3-6). Many studies have reported that high-tie IMA significantly reduces perfusion of the proximal limb of anastomosis; where its supply depend entirely on the middle colic vessels and the marginal arcade (7-9). In elderly patients with atherosclerosis or microangiopathy, transection of the LCA trunk could lead to compromised vascularity of the proximal limb of colon forming the anastomosis; particularly when the marginal artery is absent at the splenic flexure (10).
Low-tie IMA helps to maintain adequate perfusion to the proximal limb of anastomosis, avoids extensive dissection at the root of IMA and thus minimizes the risk for ANI (11). In 1981, Heald introduced total mesorectal excision (TME) as the ‘gold standard’ for rectal cancers (12). Later the concepts of TME and neoadjuvant therapy have brought significant improvements in the rectal cancer treatment outcomes. Neoadjuvant treatment has shown the potential to sterilize the IMA node metastasis (13). Several studies comparing high-tie with low-tie have reported a stage-specific survival benefit for high-tie; for those patients with involved nodes at the IMA root (14,15). Moreover, rectal cancer can have alternate lymphatic drainage; tumors of the upper rectum may drain via the lymphatic channels along the portal vein and tumours of the lower rectum may drain through lateral ligament lymphatics to the iliac nodes (16). Thus a high-tie IMA for lymph node clearance might not give the intended benefits of extended lymphadenectomy.
In laparoscopic rectal resection (LRR) many centres prefer routine high-tie IMA because of its technical ease and to ensure adequate length of proximal colon for anastomosis. Many studies have shown that critical length of the proximal limb is not an issue in low-tie strategy, thus routine mobilization of the splenic flexure is not indicated (7,17,18). A low-tie IMA, without mobilization of the splenic flexure decreases the complexity of the laparoscopic procedure and could reduces the operating time. However, there is scarcity of data in the literature regarding laparoscopic low-tie IMA rectal resection with TME reporting its post-surgical and oncological outcomes.
In our centre, standard open rectal resection (ORR) is done with low-tie IMA, TME and selective D3 lymphadenectomy, without routine splenic flexure mobilization, by protocol, is observed to maintain comparable surgical and oncological outcomes. The same procedure is thus being followed in our LRR as well.
To compare the post-surgical complications and oncological clearance in LRR and ORR for carcinoma rectum, done with low-tie IMA, TME and selective D3 lymphadenectomy.
Methods
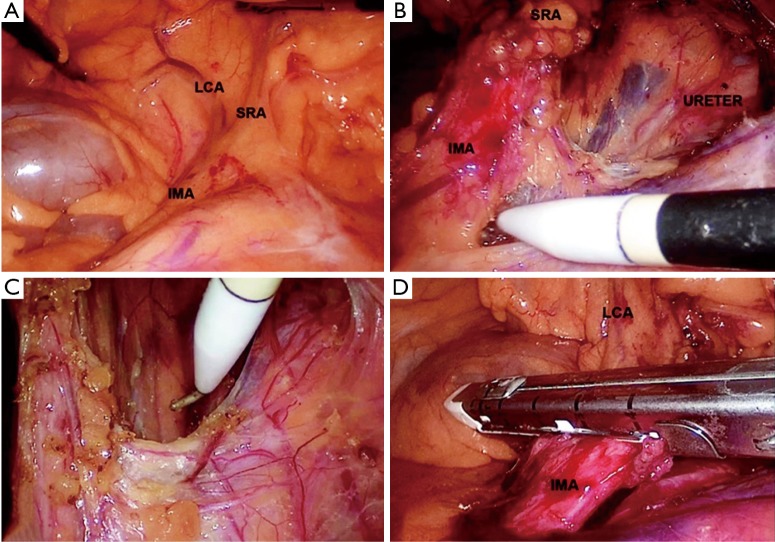
This was designed as a single centre retrospective study, based on the prospectively maintained department database of operated cases of biopsy proven carcinoma rectum or rectosigmoid (up to 20 cm from anal verge), managed with laparoscopic or open resection, done with low-tie IMA and TME without splenic flexure mobilisation, between April, 2014 and March, 2017. During the surgery; IMA was identified, traced to SRA and was ligated just distal to the origin of LCA (Figures 1-3) at the level of the proximal SRA. Selective D3 lymphadenectomy (19) was done if there were enlarged lymph nodes at the IMA root detected intraoperatively or in the pre-operative imaging. Those cases of carcinoma with associated diseases (polyposis coli/inflammatory bowel disease/synchronous malignancy), complete response to neoadjuvant chemoradiation (NACRT), Hartmann’s procedures, leak test positivity and those cases which were converted before proximal vascular ligation were excluded from this study.
Figure 1.
LRR—low-tie IMA. (A) Identification of IMA, SRA and LCA; (B) IMA root lymphadenectomy; (C) preserving autonomic nerve plexus; (D) low division of IMA. LRR, laparoscopic rectal resection; IMA, inferior mesenteric artery; SRA, superior rectal artery; LCA, left colic artery.
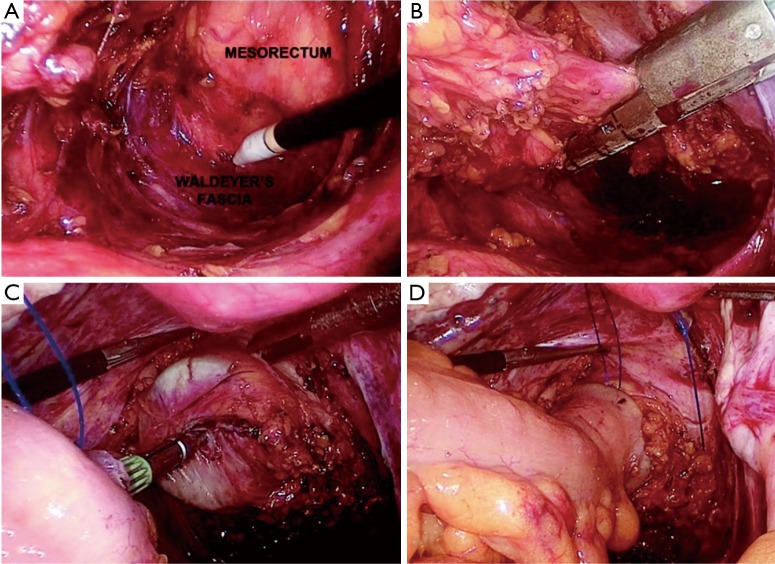
Figure 2.
LRR—TME and double stapler anastomosis. (A) TME; (B) transection of rectum; (C) anastomosis; (D) proximal limb of anastomosis. TME, total mesorectal excision.
Figure 3.

Extent of resection. (A) Extent of resection; (B) resected specimen.
The patients were grouped into LRR and ORR. The choice of the procedure was taken preoperatively based on the patient factors on the pre-operative evaluation. A retrospective analysis of the two groups was done comparing the demographic data, clinical details, investigation reports, operative data, postoperative complications and the histopathological characteristics. The socio-demographic variables included were age and gender. Clinical details studied were symptoms, duration of symptoms, NACRT status, comorbidities and distance of the lesion from the anal verge (in cm). Investigation details analysed were preoperative CEA level (in ng/mL), biopsy and clinical TNM (cTNM) stage from CECT/MRI. Operative data included were diagnosis on exploration, surgery done, duration of surgery (in hours), type of anastomosis done, postoperative complications and Clavien-Dindo grade. Histopathological variables studied on the resected specimen included length of the specimen, pathologic TNM (pTNM) stage (NCCN guidelines Version 2.2016, AJCC Cancer Staging Manual, 7th Edition), tumour differentiation, lymph node yield, lymphovascular invasion, tumour necrosis, length of distal margin (in cm), distal margin positivity, radial margin positivity, distal doughnut positivity and IMA lymph node positivity. The study proceedings were performed in accordance with the Declaration of Helsinki. Statistical analysis was done using SPSS-20. The continuous variables were studied using mean and standard deviation and the categorical variables by proportions. Bivariate analysis was done using student-t and Pearson Chi-square tests for a statistically significant difference (P<0.05).
Results
A total number of 118 patients satisfying inclusion and exclusion criteria were enrolled into the study; 48 patients in the LRR group and 70 patients in the ORR group.
Clinical details
The two groups; LRR and ORR were found comparable (Table 1) based on their age (57.44±10.13 vs. 59.29±10.69) and gender (M/F ratio 29/19 vs. 39/31), duration (in months) of symptoms (6.75±4.48 vs. 6.74±4.18), distance of the lesion (in cm) from the anal verge (8.06±5.26 vs. 9.4±5.74), pre-operative CEA (in ng/mL) level (10.1 vs. 8.4) and clinical stage T2N1M0 or more (89.6% vs. 88.6%).
Table 1. Clinical details of patients.
| Variable | LRR (n=48) | ORR (n=70) | P value |
|---|---|---|---|
| Age (mean), years | 57.44±10.13 | 59.29±10.69 | 0.34 |
| Male/female | 29/19 | 39/31 | 0.61 |
| Symptoms, % | |||
| Bleeding per rectum | 93.80 | 80.00 | 0.03* |
| Altered bowel habits | 18.70 | 51.40 | |
| Mean duration of symptom (months) | 6.75 (SD ±4.48) | 6.74 (SD ±4.18) | 0.99 |
| Mean distance from AV (in cm) | 8.06 (SD ±5.26) | 9.4 (SD ±5.74) | 0.20 |
| Site of the lesion, n (%) | |||
| Ca low rectum (up to 5 cm) | 15 (31.25) | 21 (30.00) | 0.03* |
| Ca mid rectum (5–10 cm) | 24 (50.00) | 28 (40.00) | |
| Ca upper rectum (10–15 cm) | 5 (10.42) | 13 (18.57) | |
| Ca rectosigmoid (15–20 cm) | 4 (8.33) | 8 (11.43) | |
| Comorbidities, % | 31.30 | 60.00 | 0.02* |
| Preoperative mean CEA (ng/mL) | 10.1 | 8.4 | 0.24 |
| Clinical TNM stage, % | |||
| Stage I | 10.41 | 11.43 | 0.08 |
| Stage II | 10.42 | 14.29 | |
| Stage III | 72.92 | 64.28 | |
| Stage IV | 6.25 | 10.00 | |
| Neoadjuvant chemoradiation | 75.00 | 50.00 | 0.01* |
*, a P value less than 0.05 was taken as significant. LRR, laparoscopic rectal resection; ORR, open rectal resection.
Bleeding was the commonest symptom seen in 93.8% of LRR group and 80% of ORR group. Altered bowel habits were there in 18.7% of LRR and 51.4% of ORR. Mean duration of symptoms (in months) was similar in LRR and ORR (6.75±4.48 and 6.74±4.18). Comorbidities were there in 31.3% in the LRR group and 60% in the ORR group. Among them 46.6% in LRR group and 60% in ORR group were having diabetes mellitus. Other comorbidities including hypertension and coronary heart disease were also significantly more among those patients taken for ORR (16.7% vs. 28.6%; P=0.02). There were significant differences between these groups regarding spurious diarrhoea, comorbidities and diagnosis of carcinoma rectosigmoid (with infiltration to the adjacent viscera in 37.5%; P=0.03, (OR =2.61 95% CI, 1.05–6.4) being more in the ORR group.
NACRT of 45–50 Gy in 25–28 daily fractions over 5 weeks combined with 5-fluorouracil or oral capecitabine was received by a significant proportion of LRR group and ORR group (75% vs. 50%). Mesorectal excision by protocol could be completed in all cases in both the groups.
Operative data
The spectrum of surgical procedures (Table 2) included anterior resection (16.6% in LRR and 21.43% in ORR), low anterior resection (52.08% in LRR and 47.14% in ORR), ultra low anterior resection (8.33% in LRR and 10% in ORR) and abdominoperineal resection (22.92% in LRR and 21.43% in ORR). Around 10.41% of LRR and 11.42% of ORR required resection of adjacent viscera. The mean duration of surgery (in hours) was significantly longer for LRR than ORR (5.5±1.88 vs. 4.2±1.31). Rate of conversion in the LRR group was 15.38%; the major causes for conversion being the need for adjacent visceral resection or difficulty in deep pelvic dissection. A total of 77.08% LRR and 51.43% ORR had double stapler anastomosis. Around 43.8% of LRR and 42.9% of ORR had a covering stoma as loop ileostomy or loop transverse colostomy.
Table 2. Operative data.
| Variable | LRR | ORR | P value |
|---|---|---|---|
| Mean duration of surgery (in hours) | 5.5 (SD ±1.88) | 4.2 (SD ±1.31) | 0.007 |
| Double stapler anastomosis | 77.08% | 51.43% | 0.005 |
| Complications (overall morbidity) | 12.7% | 21.4% | 0.02 |
| Clavien-Dindo grade ≥3 | 2.1% | 2.9% | 0.22 |
No post-surgical complications (Table 2) were observed in 87.3% LRR and 78.6% ORR. The overall morbidity and mortality was significantly less after LRR than ORR (12.7% vs. 21.4%). ORR showed a higher rate of complications like respiratory infection (4.8% against none in LRR) and surgical site infection (SSI) (7.1% against 2.1% in LRR). Postoperative urinary retension was similar in two groups (4.2% in LRR and 5.71% in ORR). Meanwhile LRR group showed significantly higher rate of delayed perineal wound healing than ORR (12.5% vs. 5.7%, P=0.02). One patient in each group had relaparotomy. There was one mortality in the ORR group due to massive myocardial infarction. On applying the Clavien-Dindo grading; 2.1% among LRR and 2.9% among ORR had a grade of 3 or more.
Details of tumour status are given in Table 3. Majority of LRR and ORR had T status as T2 (39.6% vs. 57.1%) or T3 (35.4% vs. 32.9%). N1 status was there in 20.8% of LRR and in 22% of ORR. N2 disease was observed in 8.3% of LRR and 5.9% of ORR. 8.9% among LRR and 7.1% among ORR had lymphovascular invasion. Around 12.5% of LRR and 12.9% of ORR showed tumour necrosis.
Table 3. Details of histopathology.
| Variable | LRR | ORR | P value |
|---|---|---|---|
| Mean length of specimen (cm) | 18.7 (SD ±4.2) | 16.6 (SD ±5.4) | 0.06 |
| Mean No. of harvested LN | 9.2 (SD ±5.3) | 8.81 (SD ±4.6) | 0.68 |
| Mean distal margin (cm) | 2.81 (SD ±1.7) | 2.74 (SD ±1.8) | 0.84 |
| NX status | 45.83% | 42.86% | 0.93 |
| IMA lymph node positive | 4.16% | 4.28% | 0.22 |
NX status, lymph node yield less than 12.
On comparing the histopathology of the specimens from LRR and ORR (Table 3); the oncological outcomes were found comparable; including the mean length (in cm) of specimen (18.7±4.2 vs. 16.6±5.4) and the mean number of harvested lymph nodes (9.2 vs. 8.81). Optimal LN harvesting was there in 54.17% of LRR and 57.14% of ORR. Mean distal margin length (in cm) was 2.81 in LRR and 2.74 in ORR. Distal margin positivity was there in 2 (4.16%) cases of LRR and 1 (1.42%) case of ORR; in these distal doughnuts were negative. Radial margin was positive in 1 (2.08%) of LRR (received NACRT) and in 1 (1.42%) case of ORR.
On analysing the lymph node yield, NX (less than 12) was the status in 33.3% of LRR and in 53.57% of ORR. 6.2% in the LRR group and 8.6% in the ORR group had metastatic disease. Selective D3 lymphadenectomy was done by protocol in 37.5% of LRR and in 37.14% of ORR. On their Subgroup analysis; among those in the LRR group 11.11% patients had positive lymph nodes at the IMA root (4.16% of the entire cohort). In the ORR group 11.54% patients had positive lymph nodes at the IMA root (4.28% of the entire cohort).
Discussion
The first successful laparoscopic colon resection was reported in 1991 (20). Now the laparoscopic resection has become the accepted treatment option for carcinoma rectum worldwide (21). Many trials have shown that laparoscopic TME for rectal cancer is a feasible and safe procedure, having the oncologic results comparable to the open surgery and with a favorable outcome (22,23).
Here the study groups were found comparable regarding their age, duration of symptoms, pre-operative CEA level, clinical TNM staging, distance of the lesion from the anal verge and the type of surgery done. The ORR group had significantly higher rates of spurious diarrhoea, comorbidities, carcinoma rectosigmoid with suspected adjacent organ infiltration and need for upfront surgery. These factors were also taken into consideration in the preoperative decision of the surgical strategy as open procedure or laparoscopic surgery.
Mean duration of surgery (hours) was significantly longer in the LRR group. The same was reported in many other comparative studies including the COLOR II trial (24). Postoperative morbidities were less among the patients who underwent LRR compared to ORR. The complications like SSI and respiratory infection were less in the LRR than in ORR; but delayed healing of the perineal wound was significantly more in the LRR group. 75% of LRR had received NACRT which might have contributed to this. The overall morbidity and mortality in the LRR was 12.7% and in the ORR group was 21.4%. Major complications leading to relaparotomy or mortality were not different in LRR and ORR groups. COLOR II trial comparing laparoscopic and open rectal cancer surgeries reported morbidity of 40% in the laparoscopic group and 37% in the open surgery group (24).
On comparing the histopathology of the harvested specimens from the two groups; mean length of specimen, mean number of harvested lymph nodes and distal margin length were statistically comparable. Two cases in the LRR group and one in the ORR group had distal margin positivity. In all three cases the distal doughnut was negative and they had received chemoradiation preoperatively. One patient in each group had positive radial margin. The further treatment in these cases was decided according to the multidisciplinary tumour board opinion. COLOR II trial and ALaCaRT RCT have reported that there is no significant difference between laparoscopic and open rectal cancer surgery regarding CRM positivity, distal margin positivity and TME completeness (23,24). LRR was reported as feasible with acceptable outcomes after NACRT (25).
A suboptimal LN yield (NX) was seen in 45.83% among LRR and 42.86% among ORR; out of which 79.31% LRR and 51.85% ORR had NACRT. Many studies have reported that the mean lymph node retrieval after NACRT followed by surgery is significantly less than after surgery alone. Some trials have opined that the retrieval of fewer lymph nodes may be a marker of a higher tumor response and better prognosis following neoadjuvant treatment (26,27).
The rate of lymph node metastases around the root of IMA reported as 3.6% in pT3/T4 sigmoid colon cancer and 5.1% in rectal cancer (28). Selective D3 lymphadenectomy was done according to the protocol in 37.5% of LRR and 37.14% of ORR. In this study the patients with positive IMA root lymph nodes was 4.16% in the LRR group and 4.28% in the ORR group, which was comparable statistically. It is reported that the neoadjuvant treatment has the potential to sterilize metastasis in IMA nodes (13). In this study a major proportion of patients in the LRR and ORR group had neoadjuvant treatment according to their clinical tumour stage in the preoperative evaluation.
A recent comparative study states that low-tie with LND is anatomically less invasive and is not inferior to high tie from the prognostic point of view (29). We have observed that the rectal resection with low-tie IMA and selective D3 lymph node sampling was feasible in achieving critical length of the proximal anastomosing limb of the colon without routine splenic flexure mobilization. In this series of LRR with low-tie IMA, the post-surgical complications were less and the oncological clearance was comparable with the standard ORRs with low-tie IMA without routine splenic flexure mobilization.
Conclusions
The post-surgical complications and the oncological clearance were comparable in LRR and ORR done with low-tie IMA without mobilisation of splenic flexure. LRR with low-tie IMA is found technically feasible and this technique had no significant added issues regarding resection or anastomosis. A total shift to high-tie IMA with splenic flexure mobilisation is not necessary in LRR; because a simpler procedure of low-tie IMA can provide comparable surgical and oncological outcomes.
Acknowledgements
We acknowledge the co-operation and support given by the Department of Anaesthesiology, Govt. Medical College, Trivandrum.
Ethical statement: The study was approved by the Institutional Ethics committee of Government Medical College, Trivandum, Kerala (IEC NO.05/30/2017/MCT) and informed consent was taken from all the patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon. CA Cancer J Clin 1971;21:361-4. 10.3322/canjclin.21.6.361 [DOI] [PubMed] [Google Scholar]
- 2.Moynihan BG. The surgical treatment of cancer of the sigmoid flexure and rectum. With special emphasis to the principles to be observed. Surg Gynecol Obstet 1908;6:463-6. [Google Scholar]
- 3.Pramateftakis MG, Kanellos D, Vrakas G, et al. Progress in rectal cancer staging and treatment. Tech Coloproctol 2010;14 Suppl 1:S29-31. 10.1007/s10151-010-0619-7 [DOI] [PubMed] [Google Scholar]
- 4.Zhou ZG, Hu M, Li Y, et al. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc 2004;18:1211-5. 10.1007/s00464-003-9170-1 [DOI] [PubMed] [Google Scholar]
- 5.Liang JT, Huang KC, Lai HS, et al. Oncologic results of laparoscopic D3 lymphadenectomy for male sigmoid and upper rectal cancer with clinically positive lymph nodes. Ann Surg Oncol 2007;14:1980-90. 10.1245/s10434-007-9368-x [DOI] [PubMed] [Google Scholar]
- 6.Peeters KC, Tollenaar RA, Marijine CA, et al. Risk factors for anastomotic failure after local mesorectal excision of rectal cancer. Br J Surg 2005;92:211-6. 10.1002/bjs.4806 [DOI] [PubMed] [Google Scholar]
- 7.Corder AP, Karanjia ND, Williams JD, et al. Flush aortic tie versus selective preservation of the ascending left colic artery in low anterior resection for rectal carcinoma. Br J Surg 1992;79:680-2. 10.1002/bjs.1800790730 [DOI] [PubMed] [Google Scholar]
- 8.Dworkin MJ, Allen-Mersh TG. Effect of inferior mesenteric artery ligation on blood flow in the marginal artery dependent sigmoid colon. J Am Coll Surg 1996;183:357-60. [PubMed] [Google Scholar]
- 9.Seike K, Koda K, Saito N, et al. Laser Doppler assessment of the influence of division at the root of the inferior mesenteric artery on anastomotic blood flow in rectosigmoid cancer surgery. Int J Colorectal Dis 2007;22:689-97. 10.1007/s00384-006-0221-7 [DOI] [PubMed] [Google Scholar]
- 10.Lange JF, Komen N, Akkerman G, et al. Riolan’s arch: confusing, misnomer, and obsolete. A literature survey of the connection(s) between the superior and inferior mesenteric arteries. Am J Surg 2007;193:742-8. 10.1016/j.amjsurg.2006.10.022 [DOI] [PubMed] [Google Scholar]
- 11.Sato K, Inomata M, Kakisako K, et al. Surgical technique influences bowel function after low anterior resection and sigmoid colectomy. Hepatogastroenterology 2003;50:1381-4. [PubMed] [Google Scholar]
- 12.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery – the clue to pelvic recurrence? Br J Surg 1982;69:613-6. 10.1002/bjs.1800691019 [DOI] [PubMed] [Google Scholar]
- 13.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Prediction of mesorectal nodal metastases after chemoradiation for rectal cancer: results of a randomised trial: implication for subsequent local excision. Radiother Oncol 2005;76:234-40. 10.1016/j.radonc.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 14.Kanemitsu Y, Hirai T, Komori K, et al. Survival benefit of high ligation of the inferior mesenteric artery in sigmoid colon or rectal cancer surgery. Br J Surg 2006;93:609-15. 10.1002/bjs.5327 [DOI] [PubMed] [Google Scholar]
- 15.Slanetz CA, Jr, Grimson R. Effect of high and intermediate ligation on survival and recurrence rates following curative resection of colorectal cancer. Dis Colon Rectum 1997;40:1205-18. 10.1007/BF02055167 [DOI] [PubMed] [Google Scholar]
- 16.Nano M, Dal Corso H, Ferronato M, et al. Ligation of the inferior mesenteric artery in the surgery of rectal cancer: anatomical considerations. Dig Surg 2004;21:123-6. 10.1159/000077347 [DOI] [PubMed] [Google Scholar]
- 17.Pezim ME, Nicholls RJ. Survival after high or low ligation of the inferior mesenteric artery during curative surgery for rectal cancer. Ann Surg 1984;200:729-33. 10.1097/00000658-198412000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan DJ, Moynagh M, Brannigan AE, et al. Routine mobilization of the splenic flexure is not necessary during anterior resection for rectal cancer. Dis Colon Rectum 2007;50:302-7. 10.1007/10350-006-0811-z [DOI] [PubMed] [Google Scholar]
- 19.Yao HW, Liu YH. Re-examination of the standardization of colon cancer surgery. Gastroenterol Rep (Oxf) 2013;1:113-8. 10.1093/gastro/got020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooperman AM, Katz V, Zimmon D, et al. Laparoscopic colon resection: a case report. J Laparoendosc Surg 1991;1:221-224. 10.1089/lps.1991.1.221 [DOI] [PubMed] [Google Scholar]
- 21.Hartley JE, Monson JR. The role of laparoscopy in the multimodality treatment of colorectal cancer. Surg Clin North Am 2002;82:1019-33. 10.1016/S0039-6109(02)00039-7 [DOI] [PubMed] [Google Scholar]
- 22.Wu WX, Sun YM, Hua YB, et al. Laparoscopic versus conventional open resection of rectalcarcinoma: A clinical comparative study. World J Gastroenterol 2004;10:1167-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson AR, Solomon MJ, Lumley JW, et al. ALaCaRT Investigators. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015;314:1356-63. 10.1001/jama.2015.12009 [DOI] [PubMed] [Google Scholar]
- 24.van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. 10.1016/S1470-2045(13)70016-0 [DOI] [PubMed] [Google Scholar]
- 25.Jeong SY, Park JW, Nam BH. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 2014;15:767-74. 10.1016/S1470-2045(14)70205-0 [DOI] [PubMed] [Google Scholar]
- 26.de Campos-Lobato LF, Stocchi L, de Sousa JB, et al. Less than 12 nodes in the surgical specimen after total mesorectal excision following neoadjuvant chemoradiation: it means more than you think! Ann Surg Oncol 2013;20:3398-406. 10.1245/s10434-013-3010-x [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Jo JS, Lee SY, et al. Low lymph node retrieval after preoperative chemoradiation for rectal cancer is associated with improved prognosis in patients with a good tumor response. Ann Surg Oncol 2015;22:2075-81. 10.1245/s10434-014-4235-z [DOI] [PubMed] [Google Scholar]
- 28.Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal carcinoma. 8th ed. Tokyo: Kanehara & Co. Ltd, 2013. [Google Scholar]
- 29.Yasuda K, Kawai K, Ishihara S, et al. Level of arterial ligation in sigmoid colon and rectal cancer surgery. World J Surg Oncol 2016;14:99. 10.1186/s12957-016-0819-3 [DOI] [PMC free article] [PubMed] [Google Scholar]