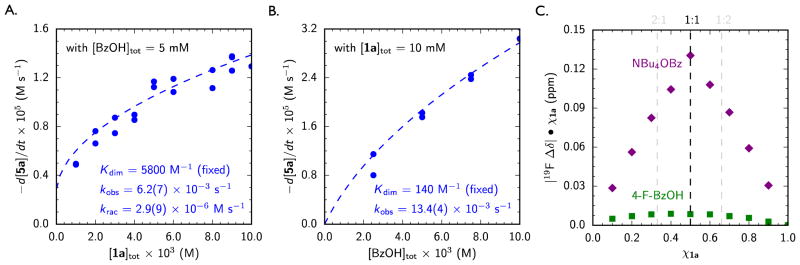
Figure 2.
Kinetic Evidence for Thiourea and Benzoic Acid Co-Catalysis. (A) The rate dependence for consumption of imine 5a (see Scheme 1) on [1a]tot. (B) The rate dependence for consumption of imine 5a on [BzOH]tot. Rates determined at 20% conversion. Blue dashed lines represent the fit to a model accounting for non-productive catalyst self-aggregation, using independently determined self-dimerization constants (Kdim). See Supporting Information for derivation. (C) Job plot of the association of thiourea 1a and a guest, either tetrabutylammonium benzoate (purple diamonds) or 4-fluorobenzoic acid (green squares), generated by monitoring the CF3 resonance in the 19F NMR spectrum of catalyst 1a in toluene-d8 at 25 °C. [1a + guest]tot = 0.01 M