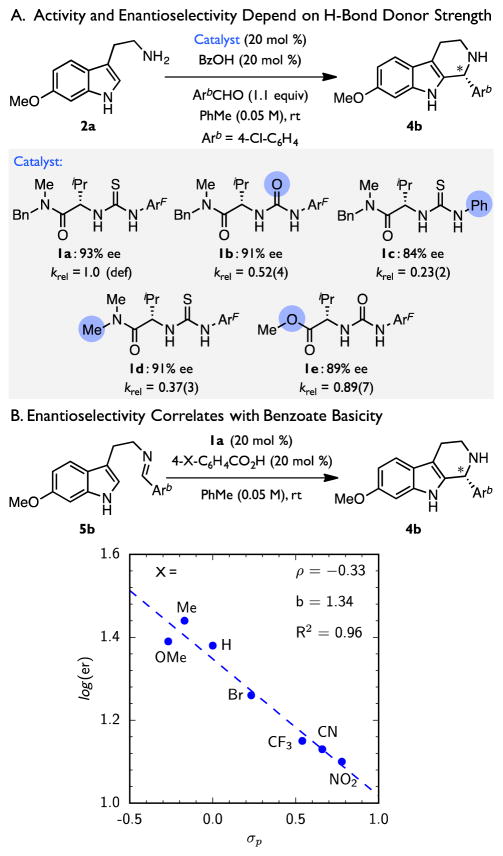
Figure 5.
Co-Catalyst Structure–Reactivity–Enantioselectivity Relationships. Enantiomeric excess reported for the formation of 4b under the conditions shown. Relative rate constants (krel) determined from the first-order rate constants for formation of 4a under the conditions in Scheme 1. Arb = 4-chlorophenyl. ArF = 3,5-bis(trifluoromethyl)phenyl.