Figure 3.
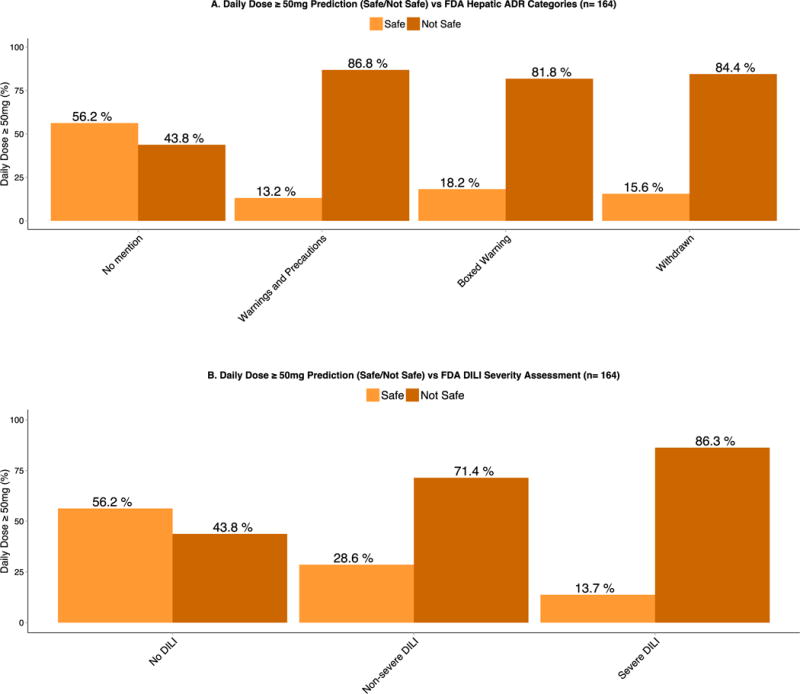
A. Daily Dose ≥ 50mg prediction (Safe/Not Safe) vs. FDA Hepatic ADR Categories.
There is a marked increase in the proportion of compounds that are dosed at greater than 50mg/day and have FDA drug label warnings associated with DILI adverse effects as illustrated in the “Warning and Precautions”, “Boxed Warning” and “Withdrawn” categories.
B. Daily Dose ≥ 50mg prediction (Safe/Not Safe) vs. FDA DILI severity assessment.
Similarly, there is a marked increase in the proportion of compounds that are dosed at greater than 50mg/day and have some type of DILI toxicity as illustrated in the “Non-severe DILI” and “Severe DILI” categories.
