Scheme 2.
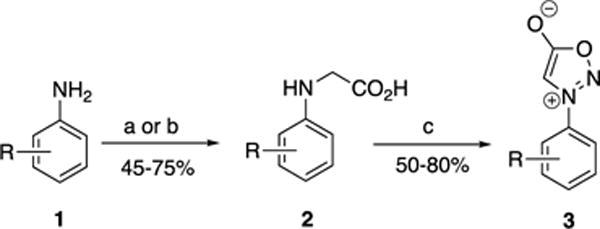
Preparation of N-arylsydnone precursors. Reagents and conditions: (a) ethyl bromoacetate, NaOAc, EtOH, reflux; LiOH, H2O:THF (1:1), 0 °C; (b) NaOAc, AcOH, glyoxylic acid monohydrate, NaBH3CN, MeOH, RT; (c) tButyl nitrite, THF, trifluoroacetic anhydride, RT. THF = tetrahydrofuran.
