Abstract
Purpose/Objective
To establish a novel preclinical model for stereotactic radiosurgery with combined mouse-like phantom quality assurance in the setting of brain metastases.
Material Methods
C57B6 mice underwent intracranial injection of B16-F10 melanoma cells. T1-post contrast MRI was performed on Day 11 after injection. The MRI images were fused with cone beam computed tomography (CBCT) images using the SARRP. Gross tumor volume (GTV) was contoured using the MRI. A single sagittal arc utilizing the 3×3 mm2 collimator was used to deliver 18 Gy prescribed to the isocenter. MRI was performed 7 days after radiation treatment and the dose delivered to the mice was confirmed using two mouse-like anthropomorphic phantoms: one in the axial and the other in the sagittal orientation. SARRP output was measured using a PTW Farmer type ionization chamber as per AAPM TG-61 and the H-D curve was generated up to a max dose of 30 Gy. Irradiated films were analyzed based on optical density distribution and H-D curve.
Results
The tumor volume at Day 11, before intervention, was 2.48±1.37 mm3 in the no SRS arm versus 3.75±1.19 mm3 in the SRS arm (NS). In the SRS arm, GTV Dose max (Dmax) and mean dose were 2048±207 and 1785±14 cGy. Using the mouse-like phantoms, the radiochromic film showed close precision as compared with projected isodose lines with a Dmax of 1903.4 and 1972.7 cGy, the axial and sagittal phantom respectively. Tumor volume 7 days post-treatment was 7.34±8.24 mm3 in the SRS arm and 60.20±40.4 mm3 in the no SRS arm (p=0.009). No mice in the control group survived more than 22 days after implantation with a median overall survival (mOS) of 19 days. mOS was not reached in the SRS group with one death noted.
Conclusion
Single fraction SRS of 18 Gy delivered in a single arc can be delivered accurately with MRI T1-post contrast based treatment planning. The mouse-like phantom allows for verification of dose delivery and accuracy.
Introduction
Brain metastases are the most common form of malignant brain tumors and radiation plays a significant role in treatment. Historically, all patients were treated with whole brain irradiation; however, with the development of Gamma Knife stereotactic radiosurgery (GKRS) and advances in linear accelerator based treatment, delivery of stereotactic radiosurgery (SRS) is a reasonable treatment option in well selected patients.[1,2] This practice has gained favor in clinical practice and improves local control in patients with brain metastases of radio-resistant histology.[3]
Preclinical models to study cancer treatment have drastically improved over the years with the development of newer, orthotopic and genetically engineered mouse models. This is true in the setting of brain metastases.[4] Similar to the advancement of preclinical models, modes of delivery of irradiation for preclinical research have dramatically improved with the development of a small animal 3D conformal irradiator for research purposes. One platform that is available is the Small Animal Radiation Research Platform (SARRP) by Xstrahl. The SARRP is a small animal irradiator that allows for 3D volumetric image guidance for localization and targeting in rodents. The system includes: 1.) On board cone-beam computed tomography (CBCT) for CT-simulation and positioning, 2.) Image fusion capability for Magnetic Resonance Imaging (MRI)-based treatment planning,[5] 3.) Advanced beam delivery including non-coplanar treatment fields and arc therapy.[6] Prior studies have demonstrated the efficacy of radiation administered using the SARRP to intracranial tumors.[5,7,8] However, the question remains whether the treatment is precise and the dose is accurate.
In this study, we hypothesized that it is feasible to accurately deliver SRS in the setting of brain metastases in a syngeneic melanoma mouse model and that the addition of radiochromic film analysis with a mouse-like phantom system can provide quality assurance for the radiation treatment. The aim of this study is to establish a melanoma intracranial orthotopic xenograft model and treat the tumor with SRS 18 Gy with MRI-based treatment planning. We used radiochromic film with the mouse-like phantom system established by Welch and colleagues to verify the treatment plan.[9] Outcomes for this study included tumor volume at 7 days after SRS and overall survival.
Material methods
Animal model
All experiments were conducted in accordance to our Institutional Animal Care and Use Committee (IACUC). 7-week old male C57BL6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were anesthetized with xylazine and ketamine for both stereotactic injections of melanoma cells as well as for delivery of SRS using the SARRP. B16-F10 cell line was purchased from ATCC and grown in DMEM with 10% FBS. A B16-F10-GFP stable cell line was created by transfecting B16-F10 cells with ready to use lentivirus expressing firefly luciferase and GFP (LVP437-PBS) from Amsbio and transfected cells were selected with puromycin. Orthotopic stereotactic injection of B16-F10 or B16-F10-GFP tumor cells were performed as described.[10,11] In brief, cells were suspended in PBS at 300 cells per μl and 1 μl of cell suspension was injected into the brain at 1 mm anterior and 2 mm lateral to the bregma. The injection needle was lowered to 2.5 mm depth from surface of the brain and cells were delivered. For survival studies, animals were monitored daily. After death, mouse brains were fixed in 4% paraformaldehyde for 48 hours and tissue sections were prepared at 5 μm thickness after which hemotoxylin and eosin stains were performed by our institutional molecular pathology shared resource.
MRI imaging and analysis
A 9.4 Tesla MRI (Bruker), located at our small animal imaging shared resource core facilities, was used to image tumor growth at 11 days status post injection and 7 days after radiation delivery (or 18 days after tumor implantation). Mice were anesthetized with isoflurane and respiratory rate was monitored throughout the procedure. 300 μl of gadolinium was injected intraperitoneally (IP) and images were obtained approximately 20 minutes after injection. T1 weighted, post contrast images were obtained and Digital Imaging and Communications in Medicine (DICOM) images were extracted. DICOM images acquired at Day 11 after injection were used for tumor targeting for SRS delivery. DICOM images acquired 7 days after radiation were analyzed with 3D-slicer software (https://www.slicer.org/) for tumor volume. Tumor volumes were contoured by trained radiation oncologists.
Stereotactic Radiosurgery
The small animal radiation research platform (SARRP) by Xstrahl (United Kingdom) was used to deliver radiation. Mice were anesthetized using xylazine and ketamine via IP injection. CBCT was obtained using the on board imager of the SARRP.[12] CBCT images were registered and MRI T1-post contrast DICOM images were fused using the MuriPlan software from Xstrahl. Fusion images were matched through anatomical features with 6-degrees of motion. The gross tumor volume was identified and contoured from the T1-post contrast image. A single-arc spanning 60 degrees was designed in the sagittal arrangement to deliver 18 Gy of radiation prescribed to the isocenter. Radiation was delivered at a potential of 220 kVp and a filament current of 13 mA. A 3×3 mm2 fixed collimator was used. Dose volume histograms were evaluated for GTV maximum, GTV minimum, GTV mean dose, and dose at 95% of the GTV volume (D95). Radiation was then delivered via the SARRP.
Physics quality assurance and mouse-like phantom
To assess for radiation delivery accuracy, two mouse-like phantoms were used.[9,13] One mouse-like phantom is in the sagittal arrangement and a second one is in the coronal arrangement. The brain of the coronal mouse-like phantom was placed in the axial spatial arrangement and will be referred to as the axial mouse-like phantom thereafter in this manuscript. CBCT was performed on both the sagittal and the axial phantom and a representative MRI T1-post contrast DICOM image was fused to the phantom CBCT images to the closest approximation. GTV was contoured using the MRI T1-post contrast image and the layer in which the radiation field would be delivered was identified. Radiochromic film, EBT3 (Ashland Advanced Materials), was placed at the center of the GTV in both the axial and sagittal phantoms. Phantoms with radiochromic film in place then underwent CBCT and MRI fusion, and a single arc in the sagittal beam arrangement was designed as per above. Following procedures outlined by the AAPM task group report 61 (TG-61), the open field output of the SARRP was measured using a PTW Farmer type ionization chamber (Model TN30013) calibrated by an accredited dosimetry calibration laboratory (ADCL).[14,15] The optical density as a function of absorbed dose (H-D curve) up to a maximum dose of 30 Gy for EBT radiochromic films was then calibrated using the ionization chamber measurements. After calibration of the radiochromic films, absolute dose distributions of irradiated films were analyzed based on the optical density distribution and H-D curve. Doses of 18 Gy were delivered to both the sagittal and axial phantom and radiochromic films were analyzed. The radiochromic film was scanned within 12 hours of delivery of radiation using an EPSON Expression 11000XL flatbed scanner with professional mode, positive film type, transparency mode, and no color correction settings. The irradiated films were scanned with a resolution of 300 dpi.
Statistics
Student T-test was used to analyze tumor volume. Kaplan Meier survival curves were used to analyze survival after intracranial implantation. Data was analyzed using SPSS (IBM). Significance was defined at p<0.05.
Results
Prior studies establishing an intracranial B16 tumor orthotopic model implanted B16 cells mixed with pre-irradiated disabled B16 cells.[16,17] To establish the intracranial orthotopic brain metastases model for melanoma without the usage of pre-irradiated disabled cells, we initially used GFP labeled B16-F10 cells (B16-F10-GFP). B16-F10-GFP cells were injected into the brain and MRI T1-post contrast series were obtained to observe tumor growth. To confirm tumor growth, mice were sacrificed and tissue sections were analyzed by H&E staining as well as with fluorescent microscopy for GFP (Figure 1A). In all subsequent experiments, wildtype B16-F10 cells were used with 300 cells in 1 μl injected into the brain. MRI T1-post contrast images were obtained at approximately 20 minutes after injection of gadolinium. Serial scans with MRI T1-post contrast were obtained at Day 11 after tumor cell injection (Figure 1B), and tumor volume analysis was obtained 7 days after intervention on Day 18.
Figure 1.
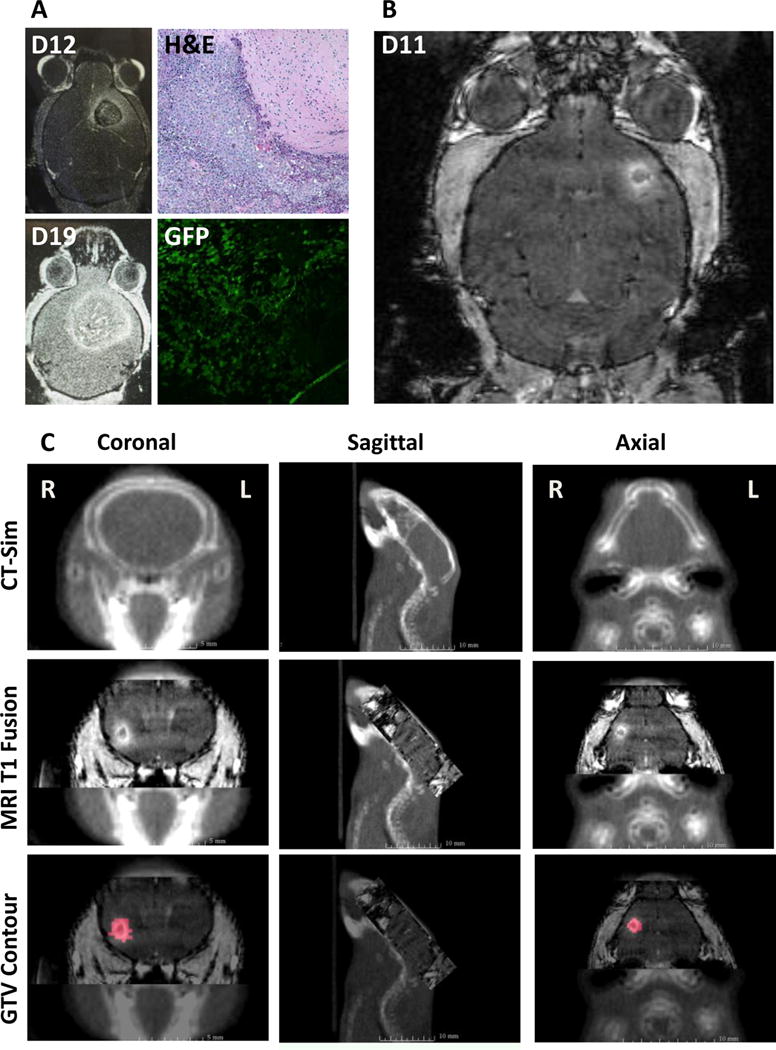
Establishing an intracranial melanoma model and MRI based radiation treatment planning. A. Intracranial B16-F10-GFP cells were used to establish the animal model. H&E staining and GFP fluorescence confirmed tumor growth. B. MRI T1-post contrast performed after 11 days of intracranial injection of B16-F10 produced small intracranial lesions. C. Gross tumor volumes (GTV) were contoured from the MRI T1-post contrast DICOM images that were fused with treatment planning cone beam CT scans.
To assess the feasibility of MRI based treatment planning using the SARRP, MRI-T1 post contrast images were obtained using 9.4 T MRI. CBCT was performed on the experimental mouse for anatomical set up. Given that the mouse was fully anesthetized, no additional stereotactic device was needed to stabilize the head and body. DICOM images were fused to the SARRP Xstrahl treatment planning software. Using lateral, posterior/inferior, anterior/posterior, pitch, jaw, and roll, the MRI images were fused to the CBCT allow for tumor visualization (Figure 1C). GTV was identified using the MRI T1-post contrast scan and a single arc beam arrangement using a 3×3 mm2 collimator with a 60 degree arc was designed for the delivery of 18 Gy (Figure 2A). In the SRS arm, GTV Dose max (Dmax), GTV minimum dose, mean dose, and D95 were 2048±207 cGy, 1690±16 cGy, 1785±14 cGy, and 1722±12 cGy respectively. A representative dose volume histogram is seen in Figure 2B.
Figure 2.
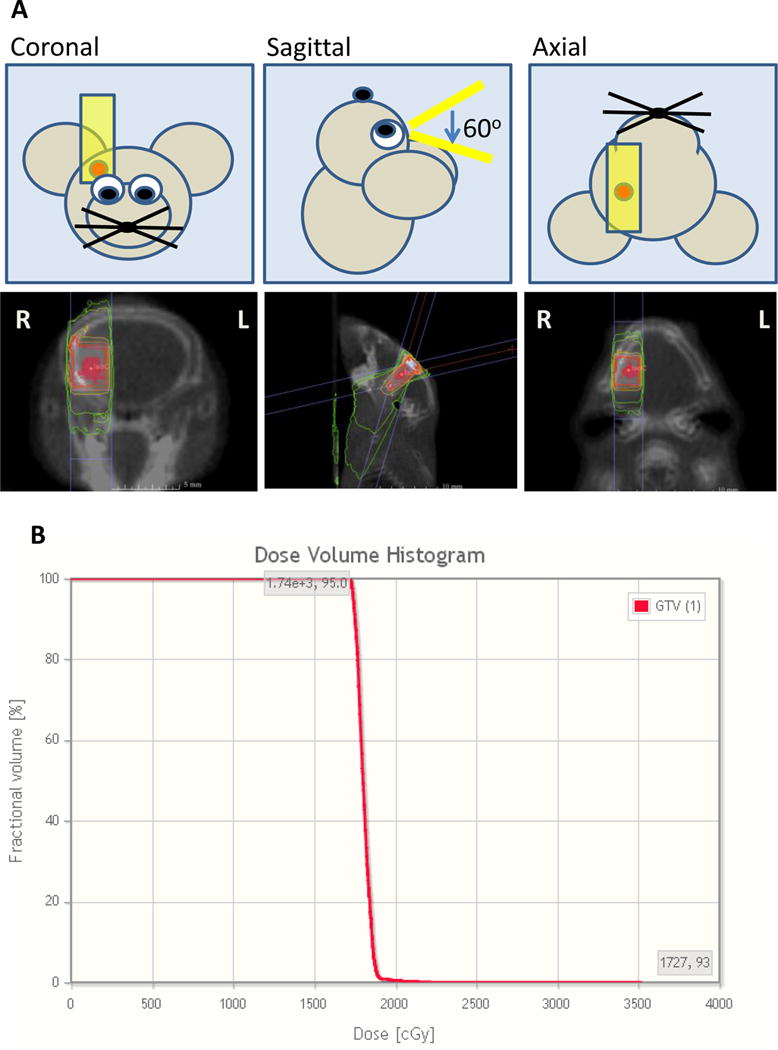
Radiation treatment planning and representative dose volume histogram. A. Single sagittal arc was planned in the sagittal orientation to minimize lateral scatter using a 3×3 collimator with a 60 degree arc. B. Representative dose volume histogram of the GTV.
In order to determine the accuracy of radiation targeting as well as to quantify actual dose delivered, mouse-like phantoms with radiochromic film were used. Dr. Welch and colleagues previously published their phantom design.[9] In brief, two separate mouse-like phantoms were created using similar densities to soft tissue and bone to simulate the mouse anatomy, one in the axial orientation and one in the sagittal orientation (Figure 3 and 4). A representative MRI T1-post contrast scan of a mouse 11 days after tumor cell injection was used to fuse with a CBCT performed on each of the phantom. Single arc 18 Gy in the sagittal orientation was planned and delivered in the same fashion as completed in the animal model above. The radiation distribution seen on the radiochromic film was similar to that of the projected isodose from the MuriPlan (Xstrahl) software. Using Matlab, isodose lines were created based on the inverse intensity projected on the radiochromic film (Figure 3E and 4E). The isodose lines were overlaid onto a scanned image of the radiochromic film with the phantom. These isodose lines were similar to the isodose lines projected by the SARRP, Xstrahl software (Figure 3B, D and 4B, D). Radiochromic film was further analyzed using H-D curves generated in our department. Film analysis showed a Dmax of 1903.4 cGy and 1972.7 cGy in the axial and sagittal phantom respectively. To determine reproducibility, three independent experiments were performed on the axial phantom mouse using three separate MRI T1-post contrast images with tumors. The average GTV Dmax on treatment planning software was 2170.0 cGy with a standard deviation of 370.4 cGy. The average field Dmax measured on film was 1911.1 cGy with a standard deviation of 79.8 cGy. There was no difference in the Dmax values (p=0.302).
Figure 3.
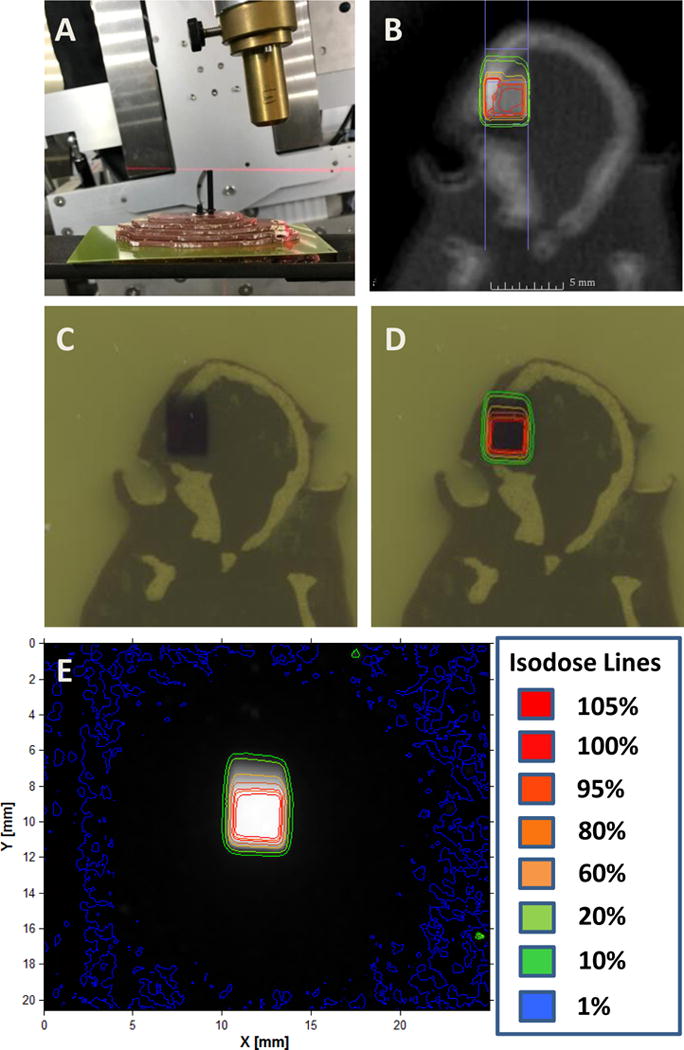
Axial mouse-like phantom based radiochromic film analysis. A. The axial phantom was used with radiochromic film placed in the approximate level of the tumor. B. Isodose lines from a single sagittal arc plan. CT-simulation was performed on the phantom and a representative mouse MRI T1-post contrast scan was used to contour the GTV. A single sagittal arc was created targeting the GTV. C. Radiochromic film after radiation delivery. D. Isodose lines generated from the radiochromic film were overlaid onto figure C. E. Isodose lines were created based on the inverse intensity projected on the radiochromic film.
Figure 4.
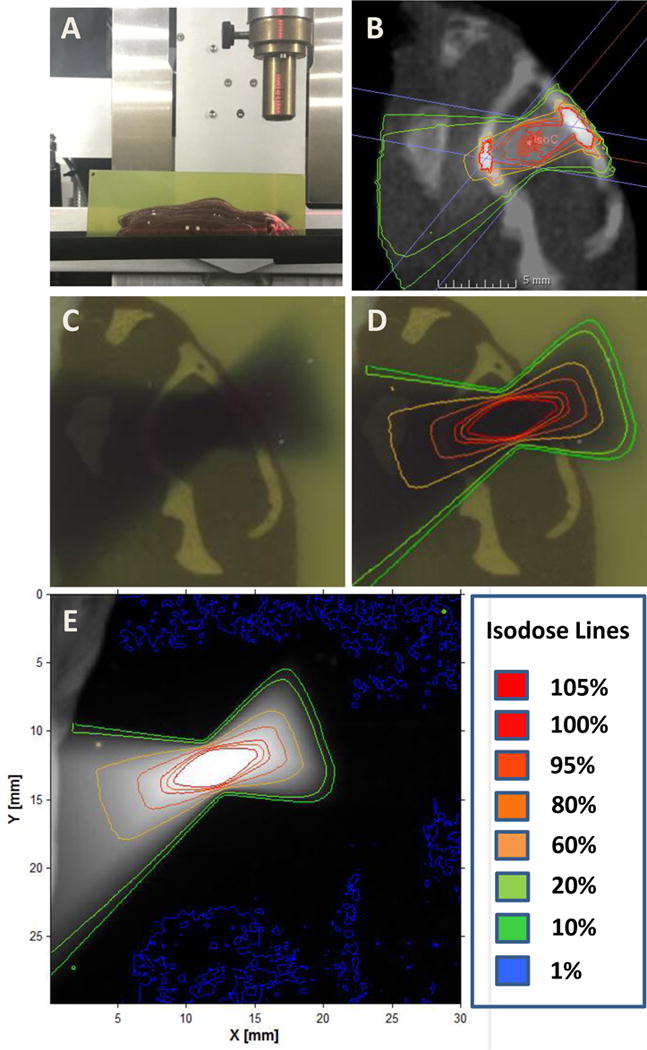
Sagittal mouse-like phantom based radiochromic film analysis. A. The sagittal phantom was used with radiochromic film placed in the approximate level of the tumor. B. Isodose lines from a single sagittal arc plan. CT-simulation was performed on the phantom and a representative mouse MRI T1-post contrast scan was used to contour the GTV. A single sagittal arc was created targeting the GTV. C. Radiochromic film after radiation delivery. D. Isodose lines generated from the radiochromic film were overlaid onto Figure C. E. Isodose lines were created based on the inverse intensity projected on the radiochromic film.
Tumor volumes were analyzed at 7 days after SRS on day 18. MRI T1-post contrast images were analyzed for volume (Figure 5). In tumors treated with SRS (n=5), there was no change in tumor growth at 7 days after radiation treatment. In the no SRS control mice (n=7), there was a significant increase in tumor size. Tumor volume 7 days post-treatment was 7.34±8.24 mm3 in the SRS arm and 60.20±40.4 mm3 in the no SRS arm (p=0.009). All mice with no SRS died. Median overall survival (mOS) of the no SRS group was 19 days. mOS was not reached in the SRS group with one death noted on day 31. All surviving mice were sacrificed on day 43 (Figure 6).
Figure 5.
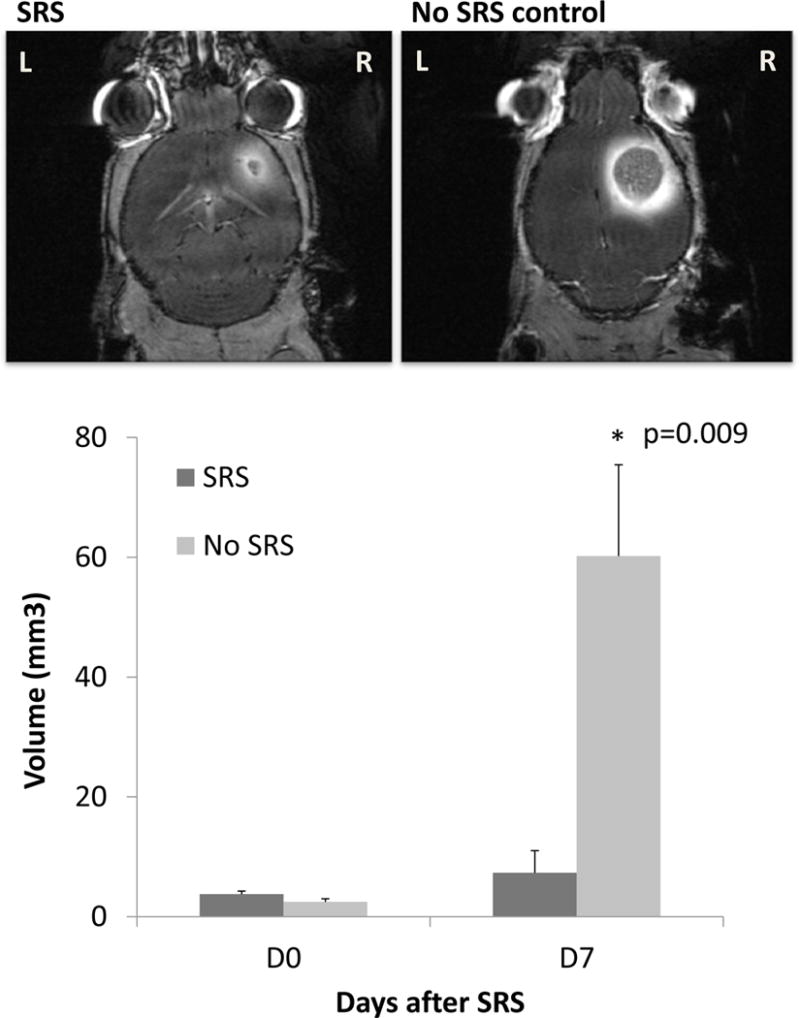
MRI T1-post contrast was obtained in mice before radiation treatment and 7 days after radiation treatment. Tumor volumes were analyzed using 3D slicer. Tumor size were smaller in the SRS mice (n=5) as compared to the no SRS control (n=7) 7 days after treatment (p=0.009).
Figure 6.
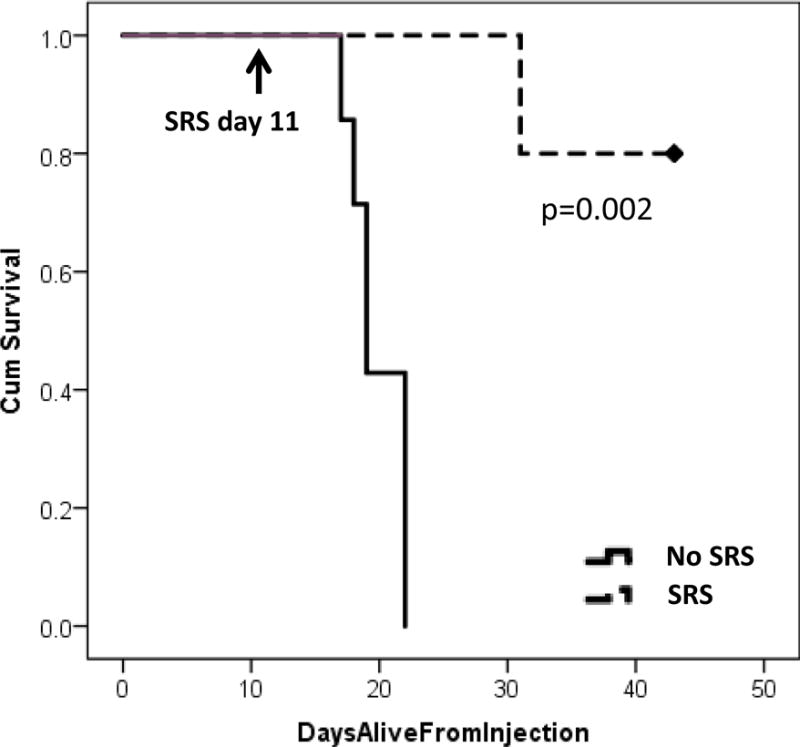
Kaplan-Meier survival curves. Mice treated with SRS (n=5) and no SRS control (n=7) had a survival difference (p=0.002).
Discussion
The treatment of brain metastases has been largely advanced with the application of SRS in patients with limited disease in the brain. This can often be achieved with Gamma Knife or linear accelerator based treatment planning. Clinical radiation doses for SRS were previously assessed by RTOG 9005 which range from 18 Gy to 24 Gy.[18] With the recent success in checkpoint inhibitor targeted immunotherapy, as well as the potential role for radiation induced abscopal effect, there has been a growing interest in studying the use of immunotherapy with SRS in the setting of brain metastases.[19,20] To be able to study these effects in a preclinical setting, a reproducible brain metastases model with accurate and precise radiation delivery as well as a way to assess dose fall off is necessary.
In this study, our goal was to establish a SRS model using the SARRP for treating melanoma brain metastases with mouse phantom quality assurance to assess radiation dose and distribution. Further, we wanted to establish a time point in which the intracranial lesion is small and more consistent with brain metastases that are treated with SRS. Prior intracranial studies using B16 melanoma syngeneic models used melanoma cells mixed with pre-irradiated disabled cells for intracranial implantation.[16,17] In contrast, this current model does not require additional pre-irradiated cells for intracranial tumor formation. Initially, we used GFP tagged B16 cell lines to develop the model; however, with the goal of adaptation to immunotherapy based research applications, all subsequent studies used wildtype B16 cells to avoid antigenicity of GFP. In designing the SRS delivery, we aimed to minimize lateral dose spillage to allow for future abscopal-based in vivo research. We elected to design our radiation field using a single sagittal arc. Although additional non-coplanar beams can be added to increase conformality, we elected not to use them to prevent lateral dose spillage.
Historically, the accuracy of radiation treatment is analyzed with in vivo mouse systems using tissue section staining for radiation effects such as γH2Ax.[21] The limitations of this technique include the usage of an animal model to detect radiation treatment ex vivo as well as the inability to assess radiation dose. Clinically, radiation treatment plans are analyzed using a phantom system. This exists in pre-clinical models as well and offers an alternative for radiation quality assurance. The mouse-like phantoms developed by Welch and colleagues is an anatomically accurate mouse phantom using 3D whole body mouse atlas created from CT data. The phantoms (one in the axial orientation and one in the sagittal orientation) are made using materials which mimic the characteristics of tissue, lung, and bone for radiation dosimetry studies.[9] These phantoms are useful in examining the accuracy and precision of radiation delivery of the SARRP. The phantom allows for: 1) Assessing the accuracy and precision of the treatment field 2) Calculating the dose delivered to radiochromic film factoring differences in tissue density, and 3) Allowing analysis of dose fall off in the non-targeted region. Furthermore, x-rays delivered by the SARRP are in the kilovoltage range as opposed to clinical linear accelerators, which are in the megavoltage range. One potential difference in the pre-clinical model for radiation delivery is the larger photoelectric effect with lower energy x-rays and how it interacts with high atomic number material in treating intracranial targets.[22]
Maximum doses measured with the sagittal and axial phantoms are in agreement with the expected doses, as are the calculated mean dose to the GTV. The isodose line distribution in the axial phantom is similar to the SARRP generated isodose lines. Interestingly, there are subtle differences in the isodose line distribution in the sagittal phantom. One difference noted is the conformality of the isodose lines in the radiochromic film as compared to the SARRP dose planning. In the SARRP dose planning, higher doses are noted in the bony structure of the phantom mouse which are not noted in the film. One possible reason for this difference is that the film is sandwiched between the sagittal layers of the phantom. Since the dose delivered is in the sagittal orientation, there is no bone tissue equivalence superior to the film to allow for scatter and dose buildup. This suggests a limitation of using the sagittal mouse-like phantom in assessing dose when radiation is delivered in a pure sagittal arrangement. At this time, we are only capable of presenting descriptive analysis as opposed to quantitative analysis. Ideally, Gamma analysis should be used to compare the projected dose to what is delivered. To our knowledge, we are not capable of exporting projected dose for Gamma analysis. Further, there are difficulties in determining the exact depth in which the film is placed to compare delivered dose versus projected dose. We are communicating with Xstrahl at this time.
Analyzing the radiation field obtained in the axial phantom orientation, the dose fall off within the treated film is rapid. The dose measured 3–5 mm lateral from the beam edge falls to within the 1–10% isodose line. This suggests that SRS delivery using the SARRP in a pre-clinical model allows for contralateral sparing and may be potentially used to assess tumor response in a bilateral intracranial injection tumor model. In our model for SRS to a single intracranial lesion, SRS to the tumor achieved local control as compared to the no SRS control. All of the mice in the no SRS control group died with a mOS 19 days. In contrast, in the SRS treated arm, one mouse was sacrificed after treatment due to disease progression. The tumor progression was noted clinically with a subdermal soft tissue mass extending from the injection site and the mouse was sacrificed as per IACUC protocol and veterinarian recommendation. The remaining mice were sacrificed approximately 7 weeks from implantation. These results are similar to the melanoma intracranial mouse model developed by Smilowitz and colleagues in which B16-F10 cells labeled with luciferase were injected into the brain mixed with disabled pre-irradiated cells.[17]
Conclusion
This is the first study to demonstrate accurate SRS delivery using the SARRP in a melanoma mouse intracranial model validated using a mouse-like phantom system. The addition of the mouse-like phantom system may provide an alternative for real-time radiation confirmation as opposed to using an ex vivo detection system.
Summary.
Single fraction SRS of 18 Gy using a single arc can be delivered accurately with MRI T1-post contrast based treatment planning to achieve local control and survival benefit. The mouse-like phantom allows for verification of dose delivery and accuracy. The addition of a phantom system provides an alternative for real-time radiation delivery confirmation as opposed to using an ex vivo detection system.
Acknowledgments
This study was supported by the ASTRO 2016 Residents/Fellows in Radiation Oncology Research Seed Grant, the Irving Institute Imaging Pilot Award, Louis V. Gerstner, Jr. Scholar Award, NIH NIAID U19-AI067773, and the NIH Biomedical Research Support Shared Instrumentation Grant 1S10OD010631-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lin X, DeAngelis LM. Treatment of brain metastases. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:3475–3484. doi: 10.1200/JCO.2015.60.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrett MD, Wu CC, Yanagihara TK, Jani A, Wang TJ. Radiation therapy for the management of brain metastases. American journal of clinical oncology. 2016;39:416–422. doi: 10.1097/COC.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 3.Yaeh A, Nanda T, Jani A, Rozenblat T, Qureshi Y, Saad S, Lesser J, Lassman AB, Isaacson SR, Sisti MB, Bruce JN, McKhann GM, 2nd, Wang TJ. Control of brain metastases from radioresistant tumors treated by stereotactic radiosurgery. Journal of neuro-oncology. 2015;124:507–514. doi: 10.1007/s11060-015-1871-5. [DOI] [PubMed] [Google Scholar]
- 4.Daphu I, Sundstrom T, Horn S, Huszthy PC, Niclou SP, Sakariassen PO, Immervoll H, Miletic H, Bjerkvig R, Thorsen F. In vivo animal models for studying brain metastasis: Value and limitations. Clinical & experimental metastasis. 2013;30:695–710. doi: 10.1007/s10585-013-9566-9. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez S, Descamps B, Vanhove C. Mri-only based radiotherapy treatment planning for the rat brain on a small animal radiation research platform (sarrp) PloS one. 2015;10:e0143821. doi: 10.1371/journal.pone.0143821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolcaen J, Descamps B, Deblaere K, Boterberg T, Hallaert G, Van den Broecke C, Decrock E, Vral A, Leybaert L, Vanhove C, Goethals I. Mri-guided 3d conformal arc micro-irradiation of a f98 glioblastoma rat model using the small animal radiation research platform (sarrp) Journal of neuro-oncology. 2014;120:257–266. doi: 10.1007/s11060-014-1552-9. [DOI] [PubMed] [Google Scholar]
- 7.Wong J, Armour E, Kazanzides P, Iordachita I, Tryggestad E, Deng H, Matinfar M, Kennedy C, Liu Z, Chan T, Gray O, Verhaegen F, McNutt T, Ford E, DeWeese TL. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. International journal of radiation oncology, biology, physics. 2008;71:1591–1599. doi: 10.1016/j.ijrobp.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armour M, Ford E, Iordachita I, Wong J. Ct guidance is needed to achieve reproducible positioning of the mouse head for repeat precision cranial irradiation. Radiation research. 2010;173:119–123. doi: 10.1667/RR1845.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welch D, Turner L, Speiser M, Randers-Pehrson G, Brenner DJ. Scattered dose calculations and measurements in a life-like mouse phantom. Radiation research. 2017 doi: 10.1667/RR004CC.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierce AM, Keating AK. Creating anatomically accurate and reproducible intracranial xenografts of human brain tumors. Journal of visualized experiments: JoVE. 2014:52017. doi: 10.3791/52017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramp TR, Camphausen K. Combination radiotherapy in an orthotopic mouse brain tumor model. Journal of visualized experiments: JoVE. 2012:e3397. doi: 10.3791/3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Armour M, Wang KK, Gandhi N, Iordachita I, Siewerdsen J, Wong J. Evaluation of a cone beam computed tomography geometry for image guided small animal irradiation. Phys Med Biol. 2015;60:5163–5177. doi: 10.1088/0031-9155/60/13/5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welch D, Harken AD, Randers-Pehrson G, Brenner DJ. Construction of mouse phantoms from segmented ct scan data for radiation dosimetry studies. Phys Med Biol. 2015;60:3589–3598. doi: 10.1088/0031-9155/60/9/3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma CM, Coffey CW, DeWerd LA, Liu C, Nath R, Seltzer SM, Seuntjens JP, American Association of Physicists in M Aapm protocol for 40–300 kv x-ray beam dosimetry in radiotherapy and radiobiology. Medical physics. 2001;28:868–893. doi: 10.1118/1.1374247. [DOI] [PubMed] [Google Scholar]
- 15.Tryggestad E, Armour M, Iordachita I, Verhaegen F, Wong JW. A comprehensive system for dosimetric commissioning and monte carlo validation for the small animal radiation research platform. Phys Med Biol. 2009;54:5341–5357. doi: 10.1088/0031-9155/54/17/017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smilowitz HM, Sasso D, Lee EW, Goh G, Micca PL, Dilmanian FA. Therapy model for advanced intracerebral b16 mouse melanoma using radiation therapy combined with immunotherapy. Cancer immunology, immunotherapy: CII. 2013;62:1187–1197. doi: 10.1007/s00262-013-1423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smilowitz HM, Micca PL, Sasso D, Wu Q, Dyment N, Xue C, Kuo L. Increasing radiation dose improves immunotherapy outcome and prolongation of tumor dormancy in a subgroup of mice treated for advanced intracerebral melanoma. Cancer immunology, immunotherapy: CII. 2016;65:127–139. doi: 10.1007/s00262-015-1772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of rtog protocol 90-05. International journal of radiation oncology, biology, physics. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 19.Cohen JV, Kluger HM. Systemic immunotherapy for the treatment of brain metastases. Frontiers in oncology. 2016;6:49. doi: 10.3389/fonc.2016.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharabi AB, Tran PT, Lim M, Drake CG, Deweese TL. Stereotactic radiation therapy combined with immunotherapy: Augmenting the role of radiation in local and systemic treatment. Oncology. 2015;29:331–340. [PMC free article] [PubMed] [Google Scholar]
- 21.Tuli R, Surmak A, Reyes J, Hacker-Prietz A, Armour M, Leubner A, Blackford A, Tryggestad E, Jaffee EM, Wong J, Deweese TL, Herman JM. Development of a novel preclinical pancreatic cancer research model: Bioluminescence image-guided focal irradiation and tumor monitoring of orthotopic xenografts. Translational oncology. 2012;5:77–84. doi: 10.1593/tlo.11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan FM, Gibbons JP. Khan’s the physics of radiation therapy. (Fifth) [Google Scholar]


