Abstract
Objective:
Few studies linking single motherhood and maternal smoking during pregnancy consider correlated risk from problem substance use beyond history of smoking and concurrent use of alcohol. In the present study, we used propensity score methods to examine whether the risk of smoking during pregnancy associated with single motherhood is the result of potential confounders, including alcohol dependence.
Method:
Data were drawn from mothers participating in a birth cohort study of their female like-sex twin offspring (n = 257 African ancestry; n = 1,711 European or other ancestry). We conducted standard logistic regression models predicting smoking during pregnancy from single motherhood at twins’ birth, followed by propensity score analyses comparing single-mother and two-parent families stratified by predicted probability of single motherhood.
Results:
In standard models, single motherhood predicted increased risk of smoking during pregnancy in European ancestry but not African ancestry families. In propensity score analyses, rates of smoking during pregnancy were elevated in single-mother relative to two-parent European ancestry families across much of the spectrum a priori risk of single motherhood. Among African ancestry families, within-strata comparisons of smoking during pregnancy by single-mother status were nonsignificant.
Conclusions:
These findings highlight single motherhood as a unique risk factor for smoking during pregnancy in European ancestry mothers, over and above alcohol dependence. Additional research is needed to identify risks, beyond single motherhood, associated with smoking during pregnancy in African ancestry mothers.
Maternal smoking during pregnancy (SDP) is a preventable cause of numerous adverse health outcomes for children (U.S. Department of Health and Human Services [USDHHS], 2014). Perinatal outcomes of SDP include intrauterine growth restriction, preterm delivery, and fetal and infant death (Dietz et al., 2010; Shah & Bracken, 2000; USDHHS, 2004). Congenital abnormalities consequent to smoking early in pregnancy have been documented as well (Hackshaw et al., 2011). Although debate continues regarding the direct or teratogenic effect of SDP on later outcomes (Knopik, 2009; Thapar & Rutter, 2009), elevated risk of disruptive behavior disorders, including attention-deficit/ hyperactivity disorder, is also observed in children whose mothers smoked during pregnancy, compared with children whose mothers abstained from smoking (e.g., D’Onofrio et al., 2008; Gaysina et al., 2013; Knopik et al., 2005).
Reducing prevalence of maternal SDP is among the national health objectives outlined most recently in Healthy People 2020 (USDHHS, 2011); however, current estimates of SDP are far from the 2020 target of fewer than 2% of pregnant women who smoke. Despite underreporting of SDP on birth certificates (Dietz et al., 1998), there are still a considerable number of women who admit to SDP: on 2014 birth certificates, for example, 8.4% of women reported smoking at some point during pregnancy, with 6.6% continuing to smoke in the third trimester (Curtin & Mathews, 2016). As part of ongoing surveillance by the Centers for Disease Control and Prevention, in 2009-2011, approximately 11% of mothers surveyed 2-6 months after childbirth reported SDP, defined as having smoked during the last 3 months of pregnancy (Tong et al., 2013).
Rates of maternal SDP are especially high among women who are unmarried at childbirth. More than three times as many unmarried as married mothers reported SDP on 2014 birth certificates (14.7% vs. 4.1%; Curtin & Mathews, 2016), which is broadly consistent with earlier research, including studies conducted outside the United States (e.g., Kiernan & Pickett, 2006; Raatikainen et al., 2005). Furthermore, among pregnant smokers, unmarried women are less likely to quit than women who are married (or cohabiting as though married; Dejin-Karlsson et al., 1996; Kiernan & Pickett, 2006; Martin et al., 2008); unmarried women are also more likely to relapse if they do quit (Martin et al., 2008). Not surprisingly, single mothers are a frequent target of smoking prevention and cessation programs, typically as part of efforts designed for low-income pregnant women (Chamberlain et al., 2013; USDHHS, 2014).
In the present study, we examined unique risk of SDP associated with single motherhood. Unmarried women face a number of challenges (McLanahan, 2009) that might separately account for and thus confound associations with prenatal smoking, including socioeconomic disadvantage (Graham et al., 2006). Compared with married mothers, on average, unmarried mothers are younger at childbirth (Hamilton et al., 2015; Shattuck & Kreider, 2013) and have lower education and income (Shattuck & Kreider, 2013), characteristics that likewise distinguish pregnant women who smoke from those who do not (Adams et al., 2008; Ebrahim et al., 2000). In addition, unmarried women are less likely than married women to receive adequate prenatal care related to financial and other barriers (Pagnini & Reichman, 2000), which is also true of pregnant smokers (Curtin & Mathews, 2016).
Although sociodemographic characteristics are routinely modeled as control variables, few studies linking single motherhood and SDP consider correlated risk from problem substance use beyond history of smoking (Martin et al., 2008) and concurrent use of alcohol during pregnancy (Martin et al., 2008; Urquia et al., 2013). To date, potential confounding by alcohol dependence is largely unaddressed, despite elevated rates of alcohol use disorder among women who smoke during pregnancy (Agrawal et al., 2008; Fergusson et al., 1998; Knopik et al., 2005, 2006) and among unmarried and separated or divorced parents, notably mothers (Waldron et al., 2013).
To examine whether single motherhood confers unique risk for smoking during early and later pregnancy, above and beyond shared risks associated with alcohol dependence, we conducted a propensity score analysis (PSA; Imbens & Rubin, 2015; Rosenbaum & Rubin, 1983). In PSA of non-experimental data, groups are matched on important predictors or covariates in the language of PSA. The purpose of matching is to reduce bias from confounders (see Rubin & Rosenbaum, 1985), particularly those likely to be overrepresented in certain groups, such as alcohol dependence among single mothers who smoke during pregnancy. To the extent that single mothers and mothers in two-parent families are observed to be well matched across predictors, where covariate balance is achieved, our confidence in comparisons of single and two-parent families is increased. Where matching on covariates cannot be achieved, the relationship between single motherhood and maternal SDP may be spurious, due to differences predating pregnancy or childbirth, including alcohol use disorder.
Method
Participants
Data were drawn from twin-families participating in the Missouri Adolescent Female Twin Study (MOAFTS; Heath et al., 1999, 2002; Waldron et al., 2013). MOAFTS is a prospective study targeting the total cohort of female like-sex twin pairs born in Missouri to Missouri-resident parents, identified from birth records, for the period July 1, 1975-June 30, 1985 [n = 370 African ancestry (AA), 1,999 European or other ancestry (EA) pairs]. A cohort-sequential sampling design was used. Initial cohorts of 13-, 15-, 17-, and 19-year-old twins and their families were recruited during the first 2 years of data collection, with continued recruitment of 13-year-olds in years 3 through 4. Baseline parental interviews were conducted at twins’ median age 15 (Wave 1). All participants gave verbal consent (assent if minors) following procedures approved by the Institutional Review Board at Washington University.
Analyses were limited to interview data obtained from biological mothers of twins. Of 1,738 EA and 264 AA mothers completing Wave 1 interviews, 1,714 (99%) and 260 (98%) had data on SDP, parental relationship status at childbirth, maternal and paternal histories of alcohol dependence, and relevant sociodemographic covariates. Six (3 EA, 3 AA) families were excluded from analyses because the biological father of the twins died before twins’ birth or a nonbiological father figure (e.g., stepfather, adoptive father) was present at twins’ birth; these exclusions reduced the sample to 1,711 EA and 257 AA mothers. At Wave 1, EA and AA mothers ranged in age from 25 to 64 years, with median ages 41 and 40, respectively. Additional sample characteristics are provided in Table 1 by race/ethnicity and single motherhood.
Table 1.
Sample characteristics, by race/ethnicity and single motherhood
| Variable | European ancestry families |
African ancestry families |
||||
| Two parent |
Single mother |
Two parent |
Single mother |
|||
| (n = 1,637) | (n = 74) | (n = 179) | (n = 78) | |||
| Smoking during pregnancy, n (%) | ||||||
| First trimester | 415 (25%) | 34 (46%)* | 45 (25%) | 17 (22%) | ||
| Beyond first trimester | 330.(20%) | 32 (43%)* | 36.(20%) | 17 (22%) | ||
| Covariates | ||||||
| Parental alcohol dependence symptoms,a M (SD) | ||||||
| Maternal | 0.08 (0.37) | 0.24 (0.62)* | 0.07 (0.34) | 0.12 (0.43) | ||
| Paternal | 0.82 (0.96) | 0.97 (1.02) | 0.71 (0.95) | 0.71 (0.97) | ||
| Education, in years, M (SD) | 13.38 (2.08) | 12.32 (2.06)* | 13.23 (1.89) | 12.21 (1.64)* | ||
| Age at childbirth, M (SD) | 26.91 (4.82) | 22.88 (5.12)* | 25.81 (4.96) | 23.11 (5.35)* | ||
| Year of birth, M (SD) | 1953.97 (5.47) | 1959.03 (5.43)* | 1955.34 (5.22) | 1957.45 (5.33)* | ||
| Parity, firstborn, n (%) | 672 (41) | 47 (64)* | 55 (31) | 32 (41) | ||
| Neighborhood socioeconomic deprivation,<i>b M</i> (SD) | 0.52 (0.27) | 0.74 (0.40)* | 1.09 (0.51) | 1.38 (0.35)* | ||
Notes: Within racial/ethnic group, single mother ≠ two parent,
< .05.
Log-transformed count of symptoms of DSM-IV alcohol dependence;
log-transformed principal component score for neighborhood socioeconomic deprivation.
Measures
Individual measures were drawn from telephone adaptations of the Semi-Structured Assessment of the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994; Hesselbrock et al., 1999), a semi-structured diagnostic interview developed for the Collaborative Study on the Genetics of Alcoholism (Begleiter et al., 1995). The SSAGA has documented validity (Hesselbrock et al., 1999), and retest and interrater reliability is excellent (Bucholz et al., 1994, 1995). At Wave 1, parents completed the SSAGA-II, the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994), update to the original DSM-III-R-based SSAGA.
Smoking during pregnancy.
Parental interviews included self-report assessment of lifetime history of smoking. Mothers who reported smoking 100 or more cigarettes were asked if they smoked while pregnant and, if so, for how many weeks or months. Consistent with prior work (Lian et al., 2016), two variables were coded: (a) whether mother smoked during the first trimester, even before knowing she was pregnant and (b) whether mother continued smoking beyond the first trimester, the latter to reflect chronicity of SDP. Mothers who never smoked or smoked fewer than 100 cigarettes in their lifetime were included in the reference group, along with mothers who smoked 100 or more cigarettes but did not smoke while pregnant.
Single motherhood.
Single motherhood was coded from maternal self-report of her relationship status at twins’ birth. Mothers who were unmarried and living separately from the twins’ father were coded as single at childbirth. Mothers who were married to or living with the twins’ biological father were coded as residing in a two-parent family.
Parental alcohol dependence symptoms.
Maternal and paternal symptoms of alcohol dependence were modeled as covariates in PSAs described below. Parental interviews included self-report assessment of lifetime history of DSM- IV alcohol dependence, with age at onset of individual symptoms (ranging from 0 to 7) also queried. For mothers, a count of alcohol dependence symptoms occurring before twins’ birth was coded. Paternal alcohol dependence symptoms were assessed using maternal reports based on an adaptation of the Family History Assessment Module (Rice et al., 1995). We analyzed maternal reports because of reduced participation by fathers (Waldron et al., 2013) and prior findings of moderate to strong agreement between self- and coparent reports of alcohol dependence (Waldron et al, 2012). Because coparent alcohol dependence was assessed without regard to temporal clustering or age at onset, a lifetime count of alcohol dependence symptoms was coded for fathers. Given significant positive skew, symptom-count measures of both maternal and paternal alcohol dependence were log-transformed.
Sociodemographic covariates.
A number of sociodemographic predictors were also modeled as covariates in PSAs: maternal educational attainment (years of schooling), maternal age at twins’ birth, maternal year of birth, parity (whether twins are mother’s firstborn), and socioeconomic deprivation of the neighborhood at the time of twins’ birth. Using common factor principal components analysis, a neighborhood (census tract) socioeconomic deprivation variable was constructed using eight items identified from 20 census tract-level socioeconomic indicators from 1980 Census data, and matched to residential addresses listed on twins’ birth records (Lian et al., 2016). Resulting composite scores for neighborhood socioeconomic deprivation ranged from -1.12 to 6.45, with higher values representing greater deprivation. Neighborhood socioeconomic deprivation was also log-transformed to correct for positive skew.
Analytic strategy
All analyses were performed in SAS Version 9.4 (SAS Institute Inc., Cary, NC) and conducted separately for EA and AA families, given racial/ethnic differences in SDP (Curtin & Mathews, 2016), family structure (Vespa et al., 2013), and their association (Perreira & Cortes, 2006). Preliminary descriptive analyses included tests of differences by single motherhood and twin-pair zygosity, the latter to identify potential limitations to the generalizability of twin data. Although identical (monozygotic) twinning occurs at random, fraternal (dizygotic) twining is modestly related to maternal age and socioeconomic status (Bulmer, 1970); thus, monozygotic-dizygotic differences would flag domains with potential limits to generalizability.
Following preliminary analyses, we estimated a standard logistic regression model predicting SDP from single motherhood. Results of the standard model provide an omnibus estimate of the association between single motherhood and SDP without regard to potential confounding. Next, PSA was conducted comparing SDP in single-mother and two-parent families across predicted probability of single motherhood. According to the Neyman-Rubin counterfactual principal (Sekhon, 2008), we are most confident about inferences from non-experimental data when sampling designs approximate the (impossible) counterfactual ideal of the same individuals evaluated simultaneously in A and not-A conditions. We approximate this ideal by matching single mother and two-parent families on a priori risk of single motherhood, not otherwise possible in the standard regression model (even with statistical adjustment; Rosenbaum & Rubin, 1985). For covariate selection, specification of the propensity score, and construction of propensity score strata, we followed recently refined procedures outlined by Imbens and Rubin (2015), summarized below.
Predicted probabilities of single motherhood were estimated from logistic regression models predicting single motherhood from maternal and paternal alcohol dependence symptom counts, sociodemographic covariates, and second- order terms. To select second-order covariates, a baseline model was estimated predicting single motherhood from maternal and paternal alcohol dependence symptoms and sociodemographic covariates. Consistent with Imbens and Rubin (2015), quadratic and interaction terms were added to the baseline model one at a time, with a likelihood ratio statistic computed to test the null hypothesis that the coefficient on the additional covariate is equal to zero. Quadratic and interaction terms leading to the largest increase in the likelihood function greater than 2.71 were retained. For EA families, three second-order covariates were retained: (a) a quadratic of maternal age at twins’ birth, (b) a two-way interaction between paternal alcohol dependence symptoms and neighborhood socioeconomic deprivation and (c) a two-way interaction between maternal year of birth and parity. For AA families, two second-order covariates were retained: (a) a quadratic of neighborhood socioeconomic deprivation and (b) a two-way interaction between paternal alcohol dependence symptoms and parity. Results of logistic regression models are provided in Supplemental Table A. As shown, for EA but not AA families, maternal alcohol dependence symptoms were associated with increased odds of single motherhood. For both EA and AA families, paternal alcohol dependence symptoms predicted reduced odds of single motherhood.
To ensure overlap in covariate distributions, we excluded or trimmed from the sample cases where there were no counterfactual matches, specifically, two-parent families with predicted probabilities less than the smallest predicted probability among single-mother families, and single-mother families with predicted probabilities greater than the largest predicted probability among two-parent families. Subsequent comparisons were conducted using the trimmed sample, which included 1,466 EA mothers (n = 72 single mother, 1,394 two-parent families) and 214 AA mothers (n = 76 single mother, 138 two-parent families). After trimming, we tested for independence of single motherhood and predicted probability of single motherhood as the number of strata or blocks was progressively increased. For each block, we conducted t tests of the null hypothesis of equality of the average linearized propensity score by single-mother status. Following Imbens and Rubin (2015), blocks were split at the median value of the propensity score until all t statistics were below 1 or where splitting a block would lead to a new block with too few observations. For EA families, this process resulted in six strata, with prevalence of single motherhood ranging from 1% (Block 1, very low predicted probability of single motherhood) to 24% (Block 6, very high predicted probability). For AA families, three blocks were sufficient to achieve within-strata independence of single motherhood and predicted probability of single motherhood. Prevalence of single motherhood in AA families ranged from 24% (Block 1, low predicted probability of single motherhood) to 61% (Block 3, high predicted probability).
Next, we tested for within-strata differences in maternal and paternal alcohol dependence symptoms and sociodemographic covariates as a function of single motherhood. Conditional on successful matching of covariates (evident from findings of nonsignificant differences), within-strata comparisons of SDP were conducted followed by omnibus tests across strata. Two logistic regression models were estimated—one predicting smoking during the first trimester of pregnancy from single motherhood and another predicting smoking beyond the first trimester from single motherhood. For both models, we conducted follow-up analyses to produce Cochran-Mantel-Haenszel estimates of the common odds ratio (OR) (pooled across strata) and the Breslow-Day test for homogeneity of ORs across strata (Hosmer & Lemeshow, 2000).
Results
Descriptive analyses
Single-mother and two-parent families differed significantly in the distribution of covariates. As shown in Table 1, single EA mothers reported more symptoms of alcohol dependence than did married or cohabiting EA mothers. Single EA and single AA mothers reported having completed less education and they were younger at childbirth, born more recently (as assessed by maternal year of birth), and more likely to live in socioeconomically deprived neighborhoods at twins’ birth compared with married or cohabiting mothers of the same race/ethnicity. In addition, single EA mothers were more likely to be first-time mothers. For both EA and AA families, twin-pair zygosity was unrelated to single motherhood and smoking beyond the first trimester of pregnancy (p > .05). For EA but not AA mothers, zygosity was predictive of smoking early in pregnancy, such that more EA mothers of fraternal twins reported smoking in the first trimester of pregnancy than did EA mothers of identical twins, χ2(1) = 5.16, p < .05.
Standard logistic regression
In EA families, single motherhood was associated with increased odds of smoking during the first trimester (OR = 2.50, 95% CI [1.56, 4.01]) and smoking beyond the first trimester (OR = 3.02, 95% CI [1.88, 4.85]). In AA families, the association between single motherhood and SDP was nonsignificant (smoking during first trimester: OR = 0.83, 95% CI [0.44, 1.57]; smoking beyond first trimester: OR = 1.11, 95% CI [0.58, 2.12]).
Propensity score analysis
Although associations between single motherhood and SDP in AA families were nonsignificant in the standard model, we proceeded with PSA to determine whether effects might be observed for a subset of covariate-matched AA mothers. Distribution of covariates in two-parent and single-mother families by predicted probability of single motherhood are provided in Supplemental Tables B and C. Across race/ethnicity, differences within strata between single-mother and two-parent families were nonsignificant (p > .05) with two exceptions: among EA families, in Blocks 1 and 2, single mothers reported fewer alcohol dependence symptoms (in fact, none) relative to mothers in two-parent families. With these exceptions, single-mother and two- parent families were well matched on maternal and paternal alcohol dependence symptoms and sociodemographic covariates in both EA and AA families.
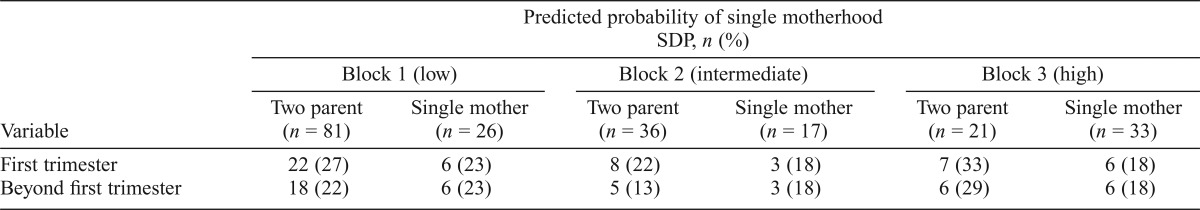
Given successful matching, within-strata comparisons of SDP by single-mother status were conducted. As shown in Table 2, with the exception of Block 6, we observed elevated rates of SDP in single-mother relative to two-parent EA families across the spectrum of a priori risk of single motherhood. In follow-up analyses, Breslow-Day tests for homogeneity of ORs across strata (first trimester: OR = 1.79, 95% CI [1.09, 2.93]; beyond first trimester: OR = 2.23, 95% CI [1.35, 3.37]) showed consistency of the association between single motherhood and SDP across strata, that is, across families stratified by predicted probability of single motherhood (first trimester: χ2[5] = 5.80, p = .33; smoking beyond the first trimester: χ2[5] = 7.62, p = .18). Among AA families, within-strata differences in rates of SDP were nonsignificant (Table 3), as were post hoc tests of the common OR.
Table 2.
Prevalence of smoking during pregnancy (SDP) in two-parent and single-mother families by predicted probability of single motherhood: European ancestry families
| Variable | Predicted probability of single motherhood SDP, n (%) |
||||||||
| Block 1 (very low) |
Block 2 (low) |
Block 3 (low intermediate) |
|||||||
| Two parent (n = 724) | Single mother (n = 9) | Two parent (n = 179) | Single mother (n = 4) | Two parent (n = 170) | Single mother (n = 13) | ||||
| First trimester | 151 (21) | 4 (44) | 45 (25) | 3 (75)† | 41 (24) | 6 (46)† | |||
| Beyond first trimester | 119 (16) | 4 (44)* | 35 (20) | 3 (75)* | 29 (17) | 6 (46)* | |||
| Block 4 (high intermediate) |
Block 5 (high) |
Block 6 (very high) |
|||||||
| Two parent | Single mother | Two parent | Single mother | Two parent | Single mother | ||||
| (n = 170) | (n =13) | (n = 81) | (n =11) | (n = 70) | (n = 22) | ||||
| First trimester | 51 (30) | 6 (46) | 38 (47) | 6 (55) | 31 (44) | 9 (41) | |||
| Beyond first trimester | 44 (26) | 6 (46)† | 27 (33) | 5 (45) | 26 (37) | 8 (36) | |||
Note: Within-block, single mother > two parent,
p < .05,
p < .10.
Table 3.
Prevalence of smoking during pregnancy in two-parent and single-mother families by predicted probability of single motherhood: African ancestry families
| Predicted probability of single motherhood SDP, n (%) |
|||||||||
| Block 1 (low) |
Block 2 (intermediate) |
Block 3 (high) |
|||||||
| Variable | Two parent | Single mother | Two parent | Single mother | Two parent | Single mother | |||
| (n = 81) | (n = 26) | (n = 36) | (n = 17) | (n = 21) | (n = 33) | ||||
| First trimester | 22 (27) | 6(23) | 8(22) | 3 (18) | 7 (33) | 6(18) | |||
| Beyond first trimester | 18 (22) | 6(23) | 5(13) | 3 (18) | 6(29) | 6(18) | |||
Discussion
Single motherhood is a well-documented predictor of maternal SDP, although few studies of unmarried pregnant smokers consider correlated risk from problem substance use. To our knowledge, the present study is the first to examine whether the effect of single motherhood on SDP is distinct from shared risks associated with alcohol dependence. In addition to standard logistic regression, to reduce bias from potential confounders, we conducted a PSA comparing rates of SDP in single-mother and two-parent families stratified by predicted probability of single motherhood.
Our findings highlight the importance of single motherhood as a unique risk factor for SDP among EA mothers, separate from alcohol dependence and highly correlated sociodemographic risk factors. In standard logistic regression models, single motherhood was associated with more than two times increased odds of SDP during the first trimester and three times increased odds of smoking beyond the first trimester. Results of PSA largely confirm the specificity of risk associated with single motherhood: with the exception of the highest probability families, increased rates of SDP were observed across the spectrum of predicted probability of single motherhood. Although it could be that EA mothers at very high risk of single motherhood will smoke regardless of relationship status, homogeneity of ORs across strata was supported, suggesting similar effects of single motherhood for EA women at varying degrees of a priori risk of single motherhood, including those at very high risk.
Among African American women, we observed little if any association between single motherhood and SDP (and symptoms of alcohol dependence), regardless of statistical approach. It is possible that single motherhood is unrelated to SDP in African American families, consistent with evidence suggesting reduced risk to African American relative to European American offspring of single mothers, because of extended familial supports but also reduced stigma (Amato, 1994). However, wide confidence intervals suggest such conclusions may be premature. Unfortunately, most studies ignore potential cultural differences, with racial/ethnic group typically modeled as a control variable if considered at all (Kiernan & Pickett, 2006; Martin et al., 2008; Page et al., 2012). Before strong inferences can be made, additional research is needed specific to African Americans. Studies of African American mothers using matching designs, based on between- or within-family contrasts (Heath et al., 2014), will necessarily require larger samples than ours for sufficiently powered analyses. This is especially true of within-family designs, where very large samples may be required to identify siblings or twins who are discordant for single motherhood.
Strengths and limitations
The present study has a number of strengths, notably use of PSA to match on parental alcohol dependence and sociodemographic risk factors, with analyses conducted separately for European and African American families. As reviewed, prior research linking single motherhood and risk of SDP has not considered potential confounding by alcohol dependence, which is predictive of both dissolution of reproductive relationships (Waldron et al., 2013) and maternal SDP (Agrawal et al., 2008; Fergusson et al., 1998; Knopik et al., 2005). Although statistical power was limited for some comparisons, results of PSA provide increased confidence in the effect of single motherhood in European American families, relative to standard models where covariate balance is not addressed. Additional strengths of the study include analyses of data drawn from a representative sample of twins and their families ascertained from state vital records, with minimal non-response by mothers. Representative sampling with high rates of participation is essential to generalize findings. However, replication in a nontwin sample including male offspring remains important given observed differences by twin zygosity.
Many limitations of the study relate to assessment. We relied on maternal report for all measures, including SDP assessed approximately 15 years after childbirth. Although reporting that is more proximal to childbirth is preferable, available evidence supports the validity and reliability of retrospective self-report of SDP, as compared with other methods and sources, including prospective self-report, multiple reporters, and biological assays (Heath et al., 2003; Klebanoff et al., 2001; Knopik et al., 2016; Pickett et al., 2009). We also relied on maternal report of paternal alcohol dependence in part to reduce bias associated with nonresponse of fathers. As a consequence, paternal alcohol dependence was assessed without regard to timing of symptom onset. Although temporal precedence remains uncertain, based on national data, we anticipate that onset of alcohol dependence symptoms will predate twins’ birth for most fathers. Among men in the United States, the average age at first diagnosis of alcohol dependence is in the early 20s, much earlier than the average age in our sample (of 28–29 years) of fathers at twins’ birth.
Future directions
Because the primary goal of the study was to examine with greater specificity risk of SDP associated with single motherhood, underlying processes were not assessed. By ruling out important confounds upstream of single motherhood, results of PSA provide a strong basis for identification of potential mediators. Among candidates, to be examined in future studies, is partner support. Single mothers are less likely to receive financial and emotional support from noncohabiting reproductive partners, compared with mothers who are married or cohabiting as though married (Carlson et al., 2004). Partner support also varies among noncohabiting couples, who may or may not continue their romantic involvement (Kiernan & Pickett, 2006; Page et al., 2012). Romantic involvement may have particular relevance for African American families, for whom we observed no effect of single motherhood on SDP and for whom part-time cohabitation, where biological parents reside together on an irregular basis, is more common (Mott, 1990); thus, romantic involvement may be a better indicator of whether African American mothers are indeed single, as opposed to measures of marriage or cohabitation.
Conclusion and implications
Maternal SDP is a known, modifiable risk behavior associated with adverse child outcomes observed prenatally onward (USDHHS, 2014). In our study, European American mothers who were unmarried and living apart from their child’s biological father were more likely than married and cohabiting mothers to smoke early and later during pregnancy, thus increasing risk to exposed children. Although European American single mothers are a potential focus of targeted smoking prevention, additional studies are needed to identify more salient predictors of maternal SDP in African American families, that is, beyond single motherhood and alcohol dependence.
Footnotes
Funding for this study was provided by National Institute on Alcohol Abuse and Alcoholism Grants AA09022, AA011998, AA017688, AA017915, and AA021492; National Institute on Drug Abuse Grant DA023134; National Cancer Institute Grant CA178331; and Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant HD049024.
References
- Adams K. E., Melvin C. L., Raskind-Hood C. L. Sociodemographic, insurance, and risk profiles of maternal smokers post the 1990s: How can we reach them? Nicotine & Tobacco Research. 2008;10:1121–1129. doi: 10.1080/14622200802123278. doi:10.1080/14622200802123278. [DOI] [PubMed] [Google Scholar]
- Agrawal A., Pergadia M. L., Waldron M., Bucholz K. K., Heath A. C., Madden P. A. F., Martin N. G. Correlates of cigarette smoking during pregnancy and its genetic and environmental overlap with nicotine dependence. Nicotine & Tobacco Research. 2008;10:567–578. doi: 10.1080/14622200801978672. doi:10.1080/14622200801978672. [DOI] [PubMed] [Google Scholar]
- Amato P. R. Life-span adjustment of children to their parents’ divorce. Future of Children. 1994;4:143–164. doi:10.2307/1602482. [PubMed] [Google Scholar]
- American Psychiatric Association. 4th ed. Washington, DC: Author; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Begleiter H., Reich T., Hesselbrock V., Porjesz B., Li T.-K., Schuckit M. A., Rice J. P. The Collaborative Study on the Genetics of Alcoholism. Alcohol Health and Research World. 1995;19(3):228–236. Retrieved from https://pubs.niaaa.nih.gov/publications/ahrw19-3/228%E2%80%93236.pdf. [PMC free article] [PubMed] [Google Scholar]
- Bucholz K. K., Cadoret R., Cloninger C. R., Dinwiddie S. H., Hesselbrock V. M., Nurnberger J. I., Jr, Schuckit M. A. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. doi:10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Bucholz K. K., Hesselbrock V. M., Shayka J. J., Nurnberger J. I., Jr, Schuckit M. A., Schmidt I., Reich T. Reliability of individual diagnostic criterion items for psychoactive substance dependence and the impact on diagnosis. Journal of Studies on Alcohol. 1995;56:500–505. doi: 10.15288/jsa.1995.56.500. doi:10.15288/jsa.1995.56.500. [DOI] [PubMed] [Google Scholar]
- Bulmer M. G. Oxford, England: Clarendon Press; 1970. The biology of twinning in man. [Google Scholar]
- Carlson M., McLanahan S., England P. Union formation in fragile families. Demography. 2004;41:237–261. doi: 10.1353/dem.2004.0012. doi:10.1353/dem.2004.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain C., O’Mara-Eves A., Oliver S., Caird J. R., Perlen S. M., Eades S. J., Thomas J. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews, Issue 10. 2013 doi: 10.1002/14651858.CD001055.pub4. Article No. CD001055 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24154953&dopt=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin S. C., Mathews T. J. Hyattsville, MD: National Center for Health Statistics; 2016, February 10. Smoking prevalence and cessation before and during pregnancy: Data from the birth certificate, 2014. National Vital Statistics Report, 65(1) Retrieved from https://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_01.pdf. [PubMed] [Google Scholar]
- Dejin-Karlsson E., Hanson B. S., Ostergren P. O., Ranstam J., Isacsson S. O., Sjöberg N. O. Psychosocial resources and persistent smoking in early pregnancy—a population study of women in their first pregnancy in Sweden. Journal of Epidemiology & Community Health. 1996;50:33–39. doi: 10.1136/jech.50.1.33. doi:10.1136/jech.50.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz P. M., Adams M. M., Kendrick J. S., Mathis M. P. Completeness of ascertainment of prenatal smoking using birth certificates and confidential questionnaires: Variations by maternal attributes and infant birth weight. American Journal of Epidemiology. 1998;148:1048–1054. doi: 10.1093/oxfordjournals.aje.a009581. doi:10.1093/oxfordjournals.aje.a009581. [DOI] [PubMed] [Google Scholar]
- Dietz P. M., England L. J., Shapiro-Mendoza C. K., Tong V. T., Farr S. L., Callaghan W. M. Infant morbidity and mortality attributable to prenatal smoking in the U.S. American Journal of Preventive Medicine. 2010;39:45–52. doi: 10.1016/j.amepre.2010.03.009. doi:10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- D’Onofrio B. M., Van Hulle C. A., Waldman I. D., Rodgers J. L., Harden K. P., Rathouz P. J., Lahey B. B. Smoking during pregnancy and offspring externalizing problems: An exploration of genetic and environmental confounds. Development and Psychopathology. 2008;20:139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim S. H., Floyd R. L., Merritt R. K., II, Decoufle P., Holtzman D.2000Trends in pregnancy-related smoking rates in the United States, 1987-1996 JAMA, 283, 361–366.doi:10.1001/jama.283.3.361 [DOI] [PubMed] [Google Scholar]
- Fergusson D. M., Woodward L. J., Horwood L. J. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Archives of General Psychiatry. 1998;55:721–727. doi: 10.1001/archpsyc.55.8.721. doi:10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- Gaysina D., Fergusson D. M., Leve L. D., Horwood J., Reiss D., Shaw D. S., Harold G. T. Maternal smoking during pregnancy and offspring conduct problems: Evidence from 3 independent genetically sensitive research designs. JAMA Psychiatry. 2013;70:956–963. doi: 10.1001/jamapsychiatry.2013.127. doi:10.1001/jamapsychiatry.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham H., Inskip H. M., Francis B., Harman J. Pathways of disadvantage and smoking careers: Evidence and policy implications. Journal of Epidemiology and Community Health. 2006;60(Supplement 2):7–12. doi: 10.1136/jech.2005.045583. doi:10.1136/jech.2005.045583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackshaw A., Rodeck C., Boniface S. Maternal smoking in pregnancy and birth defects: A systematic review based on 173 687 malformed cases and 11.7 million controls. Human Reproduction Update. 2011;17:589–604. doi: 10.1093/humupd/dmr022. doi:10.1093/humupd/dmr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton B. E., Martin J. A., Osterman M. J. K., Curtin S. C., Mathews T. J. Hyattsville, MD: National Center for Health Statistics; 2015. Births: Final data for 2014. National Vital Statistics Reports, 64(12) Retrieved from http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_12.pdf. [PubMed] [Google Scholar]
- Heath A. C., Knopik VS., Madden P. A., Neuman R. J., Lynskey M. J., Slutske W. S., Martin N. G. Accuracy of mothers’ retrospective reports of smoking during pregnancy: comparison with twin sister informant ratings. Twin Research and Human Genetics. 2003;6:297–301. doi: 10.1375/136905203322296656. doi:10.1375/136905203322296656. [DOI] [PubMed] [Google Scholar]
- Heath A. C., Madden P. A. F., Bucholz K. K. Ascertainment of a twin sample by computerized record matching, with assessment of possible sampling biases. Behavior Genetics. 1999;29:209–219. doi:10.1023/A:1021634021784. [Google Scholar]
- Heath A. C., Howells W., Bucholz K. K., Glowinski A. L., Nelson E. C., Madden P. A. F. Ascertainment of a mid-western US female adolescent twin cohort for alcohol studies: Assessment of sample representativeness using birth record data. Twin Research and Human Genetics. 2002;5:107–112. doi: 10.1375/1369052022974. doi:10.1375/1369052022974. [DOI] [PubMed] [Google Scholar]
- Heath A. C., Waldron M. C., Martin N. G., Nelson E. C., Bucholz K. K., Madden P. A. F. Human mate selection and addiction: A conceptual critique. Behavior Genetics. 2014;44:419–426. doi: 10.1007/s10519-014-9669-3. doi:10.1007/s10519-014-9669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock M., Easton C., Bucholz K. K., Schuckit M., Hesselbrock V. A validity study of the SSAGA-a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. doi:10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hosmer D. W., Lemeshow S. 2nd ed. New York, NY: John Wiley & Sons; 2000. Applied logistic regression. [Google Scholar]
- Imbens G. W., Rubin D. B. New York, NY: Cambridge University Press; 2015. Causal inference for statistics, social, and biomedical sciences: An introduction. [Google Scholar]
- Kiernan K., Pickett K. E. Marital status disparities in maternal smoking during pregnancy, breastfeeding and maternal depression. Social Science & Medicine. 2006;63:335–346. doi: 10.1016/j.socscimed.2006.01.006. doi:10.1016/j. socscimed.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Klebanoff M. A., Levine R. J., Morris C. D., Hauth J. C., Sibai B. M., Curet L. B., Wilkins D. G. Accuracy of self-reported cigarette smoking among pregnant women in the 1990s. Paediatric and Perinatal Epidemiology. 2001;15:140–143. doi: 10.1046/j.1365-3016.2001.00321.x. doi:10.1046/j.1365-3016.2001.00321.x. [DOI] [PubMed] [Google Scholar]
- Knopik V. S. Maternal smoking during pregnancy and child outcomes: Real or spurious effect? Developmental Neuropsychology. 2009;34:1–36. doi: 10.1080/87565640802564366. doi:10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik V. S., Heath A. C., Jacob T., Slutske W. S., Bucholz K. K., Madden P. A. F., Martin N. G. Maternal alcohol use disorder and offspring ADHD: Disentangling genetic and environmental effects using a children-of-twins design. Psychological Medicine. 2006;36:1461–1471. doi: 10.1017/S0033291706007884. doi:10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- Knopik S. V., Marceau K., Palmer R. H., Smith T. F., Heath A. C. Maternal smoking during pregnancy and offspring birth weight: A genetically-informed approach comparing multiple raters. Behavior Genetics. 2016:353–364. doi: 10.1007/s10519-015-9750-6. doi:10.1007/s10519-015-9750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik V. S., Sparrow E. P., Madden P. A. F., Bucholz K. K., Hudziak J. J., Reich W., Heath A. C. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: A female twin study. Psychological Medicine. 2005;35:625–635. doi: 10.1017/s0033291704004155. doi:10.1017/S0033291704004155. [DOI] [PubMed] [Google Scholar]
- Lian M., Madden P. A., Lynskey M. T., Colditz G. A., Lessov-Schlaggar C. N., Schootman M., Heath A. C. Geographic variation in maternal smoking during pregnancy in the Missouri Adolescent Female Twin Study (MOAFTS) PLoS ONE. 2016;11(4):e0153930. doi: 10.1371/journal.pone.0153930. doi:10.1371/journal.pone.0153930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. T., McNamara M., Milot A., Bloch M., Hair E. C., Halle T. Correlates of smoking before, during, and after pregnancy. American Journal of Health Behavior. 2008;32:272–282. doi: 10.5555/ajhb.2008.32.3.272. doi:10.5993/AJHB.32.3.5. [DOI] [PubMed] [Google Scholar]
- McLanahan S. Fragile families and the reproduction of poverty. Annals of the American Academy of Political and Social Science. 2009;621:111–131. doi: 10.1177/0002716208324862. doi:10.1177/0002716208324862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott F. L. When is a father really gone? Paternal-child contact in father-absent homes. Demography. 1990;27:499–517. doi:10.2307/2061567. [PubMed] [Google Scholar]
- Page R. L., Padilla Y. C., Hamilton E. R. Psychosocial factors associated with patterns of smoking surrounding pregnancy in fragile families. Maternal and Child Health Journal. 2012;16:249–257. doi: 10.1007/s10995-010-0735-z. doi:10.1007/s10995-010-0735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnini D. L., Reichman N. E. Psychosocial factors and the timing of prenatal care among women in New Jersey’s HealthStart program. Family Planning Perspectives. 2000;32:56–64. doi:10.2307/2648213. [PubMed] [Google Scholar]
- Perreira K. M., Cortes K. E. Race/ethnicity and nativity differences in alcohol and tobacco use during pregnancy. American Journal of Public Health. 2006;96:1629–1636. doi: 10.2105/AJPH.2004.056598. doi:10.2105/AJPH.2004.056598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett K. E., Kasza K., Biesecker G., Wright R. J., Wakschlag L. S. Women who remember, women who do not: A methodological study of maternal recall of smoking in pregnancy. Nicotine & Tobacco Research. 2009;11:1166–1174. doi: 10.1093/ntr/ntp117. doi:10.1093/ntr/ntp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raatikainen K., Heiskanen N., Heinonen S. Marriage still protects pregnancy. BJOG: An International Journal of Obstetrics and Gynaecology. 2005;112:1411–1416. doi: 10.1111/j.1471-0528.2005.00667.x. doi:10.1111/j.1471-0528.2005.00667.x. [DOI] [PubMed] [Google Scholar]
- Rice J. P., Reich T., Bucholz K. K., Neuman R. J., Fishman R., Rochberg N., Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. doi:10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P. R., Rubin D. B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi:10.1093/biomet/70.1.41. [Google Scholar]
- Rosenbaum P. R., Rubin D. B. The bias due to incomplete matching. Biometrics. 1985;41:103–116. doi:10.2307/2530647. [PubMed] [Google Scholar]
- Sekhon J. S. The Neyman-Rubin model of causal inference and estimation via matching methods. In: Box-Steffensmeier J., Brady H., Collier D., editors. The Oxford handbook of political methodology. Oxford, England: Oxford University Press; 2008. pp. 271–299. [Google Scholar]
- Shah N. R., Bracken M. B. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. American Journal of Obstetrics and Gynecology. 2000;182:465–472. doi: 10.1016/s0002-9378(00)70240-7. doi:10.1016/S0002-9378(00)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck R. M., Kreider R. M. Social and economic characteristics of currently unmarried women with a recent birth: 2011. American Community Survey Reports. 2013 Retrieved from http://www.census.gov/prod/2013pubs/acs-21.pdf. [Google Scholar]
- Thapar A., Rutter M. Do prenatal risk factors cause psychiatric disorder? Be wary of causal claims [Editorial] British Journal of Psychiatry. 2009;195:100–101. doi: 10.1192/bjp.bp.109.062828. doi:10.1192/bjp.bp.109.062828. [DOI] [PubMed] [Google Scholar]
- Tong V. T., Dietz P. M., Morrow B., D’Angelo D. V., Farr S. L., Rockhill K. M., England L. J. Trends in smoking before, during, and after pregnancy — Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000-2010. MMWR Surveillance Summaries. 2013, November 08;62(SS-6):1–19. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6206a1.htm. [PubMed] [Google Scholar]
- Urquia M. L., O’Campo P. J., Ray J. G. Marital status, duration of cohabitation, and psychosocial well-being among childbearing women: A Canadian nationwide survey. American Journal of Public Health. 2013;103(2):e8–e15. doi: 10.2105/AJPH.2012.301116. doi:10.2105/AJPH.2012.301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Atlanta, GA: Author; 2004. The health consequences of smoking: A report of the Surgeon General, 2004. Retrieved from http://www.cdc.gov/tobacco/data_statistics/sgr/2004/complete_report/index.htm. [Google Scholar]
- U.S. Department of Health and Human Services. Washington, DC: Author; 2011. Healthy People 2020. Retrieved from http://www.healthy-people.gov/ [Google Scholar]
- U.S. Department of Health and Human Services 2014The health consequences of smoking—50 years of progress: A report of the Surgeon General, 2014 .Atlanta, GA: Author; Retrieved from https://www.surgeongeneral.gov/library/reports/50-years-of-progress/ [Google Scholar]
- Vespa J., Lewis J. M., Kreider R. M. Washington, D.C: U.S. Census Bureau; 2013, August. America’s families and living arrangements: 2012. Population characteristics, P20-570. Retrieved from http://www.census.gov/prod/2013pubs/p20–570.pdf. [Google Scholar]
- Waldron M., Bucholz K. K., Lynskey M. T., Madden P. A. F., Heath A. C. Alcoholism and timing of separation in parents: Findings in a midwestern birth cohort. Journal of Studies on Alcohol and Drugs. 2013;74:337–348. doi: 10.15288/jsad.2013.74.337. doi:10.15288/jsad.2013.74.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron M., Madden P. A. F., Nelson E. C., Knopik V. S., Glowinski A. L., Grant J. D., Heath A. C. The interpretability of family history reports of alcoholism in general community samples: Findings in a midwestern U.S. twin birth cohort. Alcoholism: Clinical and Experimental Research. 2012;36:1091–1098. doi: 10.1111/j.1530-0277.2011.01698.x. doi:10.1111/j.1530-0277.2011.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]