Abstract
The islets of Langerhans are endocrine organs characteristically dispersed throughout the pancreas. During development, endocrine progenitors delaminate, migrate radially and cluster to form islets. Despite the distinctive distribution of islets, spatially localized signals that control islet morphogenesis have not been discovered. Here, we identify a radial signaling axis that instructs developing islet cells to disperse throughout the pancreas. A screen of pancreatic extracellular signals identified factors that stimulated islet cell development. These included semaphorin 3a, a guidance cue in neural development without known functions in the pancreas. In the fetal pancreas, peripheral mesenchymal cells expressed Sema3a, while central nascent islet cells produced the semaphorin receptor neuropilin 2 (Nrp2). Nrp2 mutant islet cells developed in proper numbers, but had defects in migration and were unresponsive to purified Sema3a. Mutant Nrp2 islets aggregated centrally and failed to disperse radially. Thus, Sema3a-Nrp2 signaling along an unrecognized pancreatic developmental axis constitutes a chemoattractant system essential for generating the hallmark morphogenetic properties of pancreatic islets. Unexpectedly, Sema3a- and Nrp2-mediated control of islet morphogenesis is strikingly homologous to mechanisms that regulate radial neuronal migration and cortical lamination in the developing mammalian brain.
KEY WORDS: Islet, Chemoattractant, Radial migration, Nrp2, Sema3a, Neural development, Cortex, Mouse, Human
Highlighted Article: Semaphorin signaling provides spatial information during pancreatic islet development, controlling outward radial migration of fetal islet cells similar to cortical lamination seen during neural development.
INTRODUCTION
Pancreatic islets are named for their most characteristic feature: islets are endocrine cell clusters dispersed throughout an abundant sea of exocrine tissue. The molecular and signaling origins of this conserved hallmark morphology, which are established during fetal development, remain undefined. Islets are endocrine micro-organs that are crucial for regulation of metabolism, including glucose control. Within the islets, β cells secrete insulin to stimulate glucose uptake by peripheral tissues and α cells secrete glucagon to mobilize glucose from target organs such as the liver. Islet structure may influence function: specialized vascular and neural crest-derived structures ramify within islets to regulate hormone secretion and blood flow, and interactions among islet cells can influence hormone secretion (Brissova et al., 2006; Cleaver and Dor, 2012; Lammert et al., 2003; Muñoz-Bravo et al., 2013; Reinert et al., 2014; Rodriguez-Diaz et al., 2011; van der Meulen et al., 2015). Islet morphogenesis comprises several distinct processes. Initially, islet formation begins with delamination of endocrine precursor cells from the primitive core of the branching ductal epithelium. After exiting this epithelial layer, newborn islet cells migrate away from the epithelium into the surrounding mesenchyme, cluster and begin to form recognizable islets (Benitez et al., 2012; Gouzi et al., 2011; Rukstalis and Habener, 2007). Vascularization and innervation of islets begins in the embryo and continues postnatally (Reinert et al., 2014), and islets appear to continue to disperse from large central ductal structures after birth, although this latter process has not been measured. Thus, islet morphogenesis could be parsed into delamination, migration, clustering and remodeling, with the possibility that each of these processes could be controlled by distinct signals. Despite the importance of islet structure to function, little is known regarding long- or short-range signals that control islet morphogenesis.
Prior studies have identified secreted signals controlling cell differentiation and proliferation in pancreas development (reviewed by Benitez et al., 2012; Gittes, 2009; Kim and Hebrok, 2001; Puri and Hebrok, 2010; Serup, 2012). For example, classical fetal organ culture studies showed that pancreatic mesenchyme provides cues for epithelial expansion, morphogenesis and differentiation (Gittes et al., 1996; Golosow and Grobstein, 1962; Landsman et al., 2011; Wessells and Cohen, 1967). Studies by Bhushan et al. (2001) revealed that Fgf10, which is expressed throughout the pancreatic mesenchyme at the inception of pancreas morphogenesis, is required for pancreatic progenitor proliferation. Other studies demonstrated that vascular endothelium induces foregut expression of Ptf1a and Pdx1, crucial transcriptional regulators of pancreatic growth and development (Lammert et al., 2001; Yoshitomi and Zaret, 2004). At later stages, Notch signaling – possibly through short-range lateral inhibition – controls endocrine cell specification and acinar cell differentiation from progenitor cells (Afelik et al., 2012; Apelqvist et al., 1999; Cras-Méneur et al., 2009; Esni et al., 2004; Murtaugh et al., 2003; Shih et al., 2012). Short-range signals associated with neural development, including netrins and Eph-ephrin signaling, have been associated with pancreatic cell migration, growth and islet function (Konstantinova et al., 2007; Yang et al., 2011; Yebra et al., 2003). In islet morphogenesis, extracellular signals, including EGF, HGF and TGFβ, and intracellular signal transducers, including Cdc42, Rac1, Tm4sf4 and Grg3 have been suggested to influence fetal islet morphogenesis (Anderson et al., 2011; Blum et al., 2014; Greiner et al., 2009; Guo et al., 2013; Kesavan et al., 2014; Metzger et al., 2012; Miettinen et al., 2000; Miralles et al., 1998; Pagliuca et al., 2014; Rezania et al., 2014; Sanvito et al., 1994; Tulachan et al., 2007). Thus, although many signals are known to regulate pancreas and islet development, spatially localized cues that might control islet morphogenesis have not yet been discovered.
To define signals controlling islet cell migration and movement, we designed a screen to identify secreted factors sufficient to alter β cell localization in development. We characterized one factor identified in the screen, semaphorin 3a, as a regulator of fetal islet cell migration during islet morphogenesis. The Sema3 family is composed of secreted proteins initially identified as inducers of axon growth cone collapse, and later characterized as chemoattractant or chemorepulsive factors that regulate multiple specific stages of nervous system development, including axon targeting, neuron migration and neuron polarization (Chen et al., 2008; Kolodkin et al., 1993; Polleux et al., 1998; Shelly et al., 2011; Tran et al., 2009). In addition, semaphorins have been demonstrated to function in other organ systems, including bone homeostasis and cardiovascular development (Degenhardt et al., 2013; Epstein et al., 2015; Fukuda et al., 2013; Ieda et al., 2007). Secreted semaphorins usually signal through heterodimeric receptor complexes composed of neuropilins and plexins (Chen et al., 1997; Giger et al., 2000; Kolodkin et al., 1997; Takahashi et al., 1999; Tamagnone et al., 1999; Winberg et al., 1998). Neuropilin 2 (Nrp2) expression has been reported in adult islets and Sema3a mRNA was enriched in E11.5 mouse pancreatic mesenchyme (Cohen et al., 2002; Guo et al., 2013), but a functional role for semaphorin signaling has not yet been reported in pancreatic development or physiology.
Here, we provide evidence that semaphorin signaling through Nrp2 receptors during pancreas development provides guidance cues along a previously unrecognized proximodistal axis that is essential for regulating islet morphogenesis and dispersion. This developmental signaling axis in the pancreas has striking homology to radial patterning cues required for cortical lamination during neural development, unexpectedly revealing shared use of a signaling module to establish radial pattern in the brain and pancreas.
RESULTS
A screen to identify morphogenetic signals controlling islet development
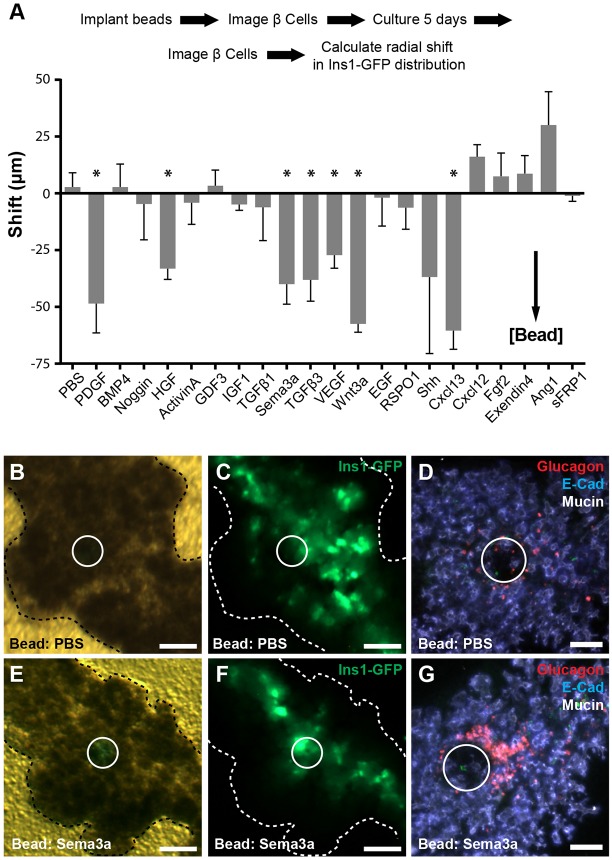
To define signals controlling islet cell migration, we identified 21 candidate secreted factors based on existing genome-wide expression datasets from fetal pancreatic mesenchyme (Guo et al., 2013) and developing islet cells (Benitez et al., 2014). To assay for effects on islet development, we implanted factor-soaked beads in cultured E13.5 Ins1-GFP transgenic mouse fetal pancreas (Fig. 1A, Fig. S1) (Hara et al., 2003). By measuring changes in the distribution of β cell-derived GFP signal around beads over 5 days of development, we identified seven growth factors that increased β cell proximity to beads (Fig. 1A). These included growth factors with established roles in islet biology, including PDGF, VEGF and HGF (Cai et al., 2012; Chen et al., 2011; Reinert et al., 2013, 2014; Roccisana et al., 2005), and ligands with unknown function in islet development, including Cxcl13 and Sema3a. Based on this β cell clustering and the pronounced α cell phenotypes described below, we chose semaphorin signaling for further study.
Fig. 1.
Development of a screen to identify secreted signals regulating islet development. (A) Schematic of bead screen workflow (see Fig. S1 for details). Results of a screen of 21 candidate signals. Statistical significance is indicated (*P<0.05, two-tailed t-test; see Table S1 for n for each signal). (B-D) Ins1-GFP fluorescence is distributed throughout the organ with PBS beads, and α cells were distributed randomly around beads, as detected by whole-mount immunofluorescence. (E,F) Ins1-GFP signal is redistributed toward the epithelial core with Sema3a-soaked beads. (G) In the distal dorsal pancreas, α cells cluster around Sema3a-soaked beads. White circles indicate bead location. Scale bars: 100 μm. Data are mean±s.e.m.
Pancreatic β cell distribution was strongly shifted toward beads soaked in Sema3a (P=0.01 relative to PBS control beads, Fig. 1B,C,E,F). We also observed clustering of glucagon+ α cells adjacent to Sema3a-soaked beads by immunofluorescence analysis (Fig. 1D,G). Quantification of α cell fluorescence relative to beads demonstrated that glucagon+ cells were significantly increased near Sema3a-soaked beads compared with PBS-soaked control beads (P=0.04, two tailed t-test: Sema3a=2348±254 versus PBS=1506±61). Many α cells lacked E-cadherin expression, consistent with acquisition of a migratory phenotype (Acloque et al., 2009). Moreover, unlike α cells in PBS controls, α cells adjacent to Sema3a-soaked beads were deep in the mesenchyme several cell diameters from nearby ductal epithelia, suggesting that Sema3a might affect islet cell migration over a relatively long range (Fig. S1). Expression of Ki67 in glucagon+ cells remained unchanged by Sema3a-soaked beads at multiple time points tested, suggesting that increased α cell proliferation did not underlie the observed phenotypes (Fig. S1). Likewise, expression of the islet progenitor marker Neurog3 was indistinguishable in organs implanted with PBS or Sema3a-soaked beads, indicating that Sema3a did not induce endocrine differentiation (Fig. S1). Other semaphorin family members, including Sema3b and Sema3f, were expressed in the pancreas at E15.5, and in bead experiments had similar effects on fetal α cell localization (Fig. S1). These data suggested that semaphorin signaling might provide guidance cues to migrating fetal islet cells.
Radial asymmetry in distribution of semaphorin signaling components in the developing pancreas
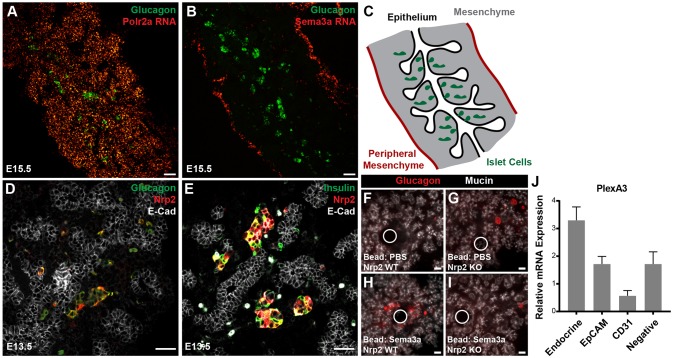
Based on established roles of semaphorins as chemoattractant or chemorepulsant guidance cues in other contexts, we next assessed whether semaphorin signaling might provide directional cues in islet morphogenesis. At E15.5, in situ hybridization revealed a striking concentration of Sema3a transcripts at the pancreatic mesenchymal periphery. By contrast, we observed uniform distribution of Polr2a transcripts encoding RNA polymerase II (Fig. 2A-C). Developing islet cells, including glucagon+ α cells, were localized to the core of the organ, adjacent to the central epithelium (Fig. 2A-C). Cells expressing Sema3a co-expressed the fibroblast marker vimentin and were enriched in FACS-purified mesenchymal cells in the fetal pancreas, supporting the view that peripheral fibroblasts expressed Sema3a (Fig. S2). We observed a similar peripheral mesenchymal localization of Sema3d using Sema3dGFP/Cre knock-in mice (Katz et al., 2012) and by measuring gene expression in FACS-purified cell populations (Fig. S2). Compared with Sema3a expression, Sema3dgfp expression appeared to extend several cell layers deeper, suggesting that a semaphorin gradient composed of multiple types of semaphorins could instruct islet morphogenesis. Alternatively, this difference in observed expression pattern could reflect differences in detecting Sema3a by in situ hybridization and Sema3d by GFP expression.
Fig. 2.
Radial asymmetry in expression of semaphorin signaling components. (A) In situ hybridization demonstrating homogenous distribution of Polr2a RNA throughout E15.5 pancreas. (B) Sema3a RNA was localized to the mesenchymal periphery of the pancreas. (C) Schematic showing orientation of epithelium, islet cells and mesenchyme. (D,E) Islet cells express Nrp2 at E13.5. (F-I) Nrp2 is necessary for α cell responses to Sema3a. (J) Quantitative PCR analysis of mRNA expression for plexin A3 in FACS-purified fetal pancreatic cell populations, relative to E15.5 whole pancreas. mRNA of the Nrp2 co-receptor Plxna3 is enriched in Neurog3gfp-positive fetal islet cells at E15.5 (P=0.03, two tailed t-test versus EpCAM+ epithelial cells, n=4 biological replicates for each group). White circles indicate bead location. Scale bars: 50 μm. Data are mean±s.e.m.
In contrast to Sema3 ligand production in peripheral mesenchyme, we detected the Sema3 receptor Nrp2 in both α and β cells at E13.5 (Fig. 2D,E). From E15.5 to adult stages, Nrp2 expression was primarily detected in α cells (Fig. S3). Nrp2 was not expressed in endocrine progenitor cells marked by Neurog3+ nuclei, suggesting expression is acquired only after differentiation into α or β cells (Fig. S3). Nrp1 was not detectable in fetal islet cells, but was present in other pancreatic cell types (Fig. S3). Human fetal α cells expressed NRP2, whereas somatostatin-expressing δ cells expressed NRP1, indicating some signaling features are conserved in human pancreas development (Fig. S3). Upon implanting Sema3a-soaked beads in cultured Nrp2 knockout mouse pancreas, we did not detect α cell aggregation around beads (Fig. 2F-I). Thus, Nrp2 is required for islet cell responses to Sema3a. These findings also indicate that other receptors like Nrp1 did not compensate for Nrp2 loss, as observed in other systems (Takashima et al., 2002).
Neuropilins act as co-receptors with plexin proteins (Takahashi et al., 1999; Tamagnone et al., 1999). Multiple mRNAs encoding plexins were detected in E15.5 mouse fetal pancreas by RT-PCR (Fig. S3). Assessment of mRNA expression of selected plexin co-receptors in FACS-purified cell populations from the E15.5 pancreas detected enrichment of Plxna3 in fetal endocrine cells relative to whole pancreas, pancreatic epithelial cell (EpCAM+), or endothelial cell subsets (CD31+; Fig. 2J). Plexins B1 and B2 were expressed in the pancreas, but were not similarly enriched in islet cells (Fig. S3). These data indicate that the expected neuropilin co-receptors are present in the appropriate cell types in the pancreas to facilitate responses to semaphorin cues. Together these findings suggest that semaphorin signals from distal mesenchyme to central Nrp2+ islet cells could define an endogenous long-range developmental axis.
Nrp2 is required for islet morphogenesis
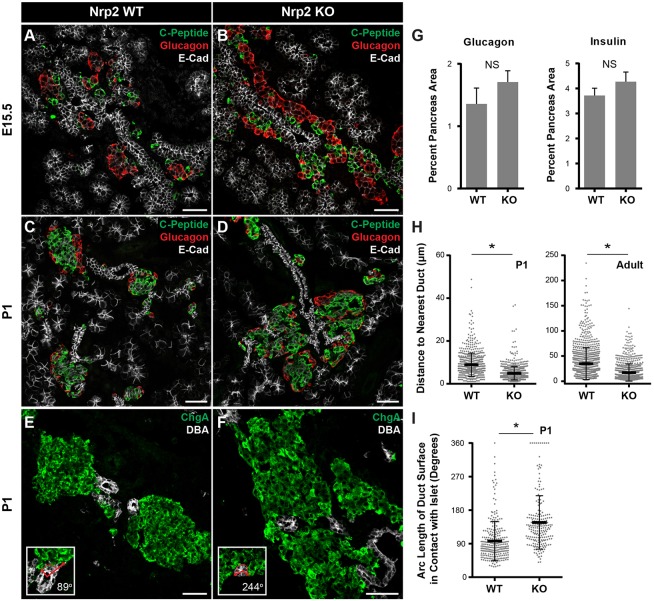
Semaphorin guidance signals can be repulsive or attractive, depending on the cellular context (Tran et al., 2007). Based on islet cell attraction to Sema3a-soaked beads and the radially asymmetric distribution of Sema3a and Sema3d transcripts, we hypothesized that semaphorins function as a chemoattractant cue for developing islet cells. If so, loss of Nrp2 should impair islet cell migration outward from their origin in the central ductal epithelium. To test this hypothesis, we assessed islet development in mice with homozygous inactivation of Nrp2 (Giger et al., 2000). Because of high-frequency perinatal mortality in homozygous Nrp2 mutants (Giger et al., 2000), we focused our analysis of pancreas development at E15.5 and postnatal day 1 (P1). At E15.5, islet cells in control littermates were distributed throughout the pancreas as discrete clusters or single islet cells, corresponding to nascent islets and cells migrating to join islets (Fig. 3A). By contrast, in E15.5 Nrp2 mutants, islet cells formed long streams of hormone+ cells along ducts (Fig. 3B). In controls at P1, islets formed as rounded structures distributed throughout the pancreas, with β cells in the islet interior surrounded by α cells (Fig. 3C). In Nrp2 mutants, we observed abnormal islet cell aggregates enveloping ductal structures in central regions of the pancreas (Fig. 3D,F). Within these aggregates, typical islet architecture appeared preserved, but individual islets were abnormally clustered near other islets and ducts. The ductal and acinar tissue in Nrp2 mutants appeared similar to controls.
Fig. 3.
Nrp2 is necessary for islet morphogenesis. (A,B) At E15.5, control islet cells are arranged in small clusters, whereas streams of islet cells are detected parallel to ducts in Nrp2 knockouts. (C-F) Islets in control P1 pancreas are round and distinct from ducts, but islets in Nrp2 knockouts surround ducts and form large islet cell aggregates. (G) The sizes of Islet α and β cell areas are unchanged in Nrp2 knockouts at P1 (P=0.32 for glucagon, P=0.28 for insulin; mean±s.e.m.). (H) Duct-islet distances are reduced in Nrp2 knockouts at P1 (P=0.026) and in 6-month-old adults (*P=0.04, data are mean±s.d.). (I) Increased contact between ductal basal surfaces and islets in Nrp2 knockouts (*P=2×10−5, data are mean±s.d.). For all quantification at P1 (H,I), n=6 for wild type and n=4 for knockout; for adult (H), n=4 for wild type and n=3 for knockout. Statistical significance was assessed using a two-tailed t-test. Insets in E,F are examples of quantification in I. Scale bars: 50 μm. Data are mean±s.d.
To quantify these phenotypes, we measured specific features of islet development and morphogenesis. Islet α cell and β cell quantities were not detectably altered in Nrp2 mutants at P1 (P=0.32, α cells; P=0.28, β cells; Fig. 3G), suggesting islet morphogenetic defects did not reflect altered proliferation or differentiation. Measurement of the distance from islets to the nearest ductal structure marked by DBA lectin indicated a 40% reduction in duct-to-islet distance in Nrp2 mutants at P1 (P=0.03, Fig. 3H). Subsequent analysis of rare surviving adult Nrp2-knockout mice indicated that this islet phenotype persisted in adults, with a halving of duct-islet distance detected (P=0.04, Fig. 3H and Fig. S4). We also quantified the extent of contact between ducts and islets and noted a significant increase in islet contact with ductal surfaces in Nrp2 mutants (97°±2 in controls, 147°±6 in mutants: P=2×10−5, Fig. 3I). Total encirclement of ducts by islets occurred rarely in controls (1.8%) compared with islets in Nrp2 mutants (8.5%, P=0.0009, Fisher's exact test). Thus, islet separation from ducts appeared to be impaired in Nrp2 mutants. We did not detect obvious alterations in islet vascularization or innervation in Nrp2 mutants (Fig. S4). To assess the requirement for Nrp2 specifically in the pancreatic islets, we attempted conditional inactivation of Nrp2 using a Cre-Lox approach. We did not observe a reduction of Nrp2 expression by immunofluorescence using either Neurog3-Cre or Pdx1-Cre (Fig. S4). Collectively, the phenotypes observed support the view that radial migration of islet cells away from their ductal origin was impaired in Nrp2 mutants.
Imaging islets in the intact pancreas of Nrp2 mutants using CLARITY
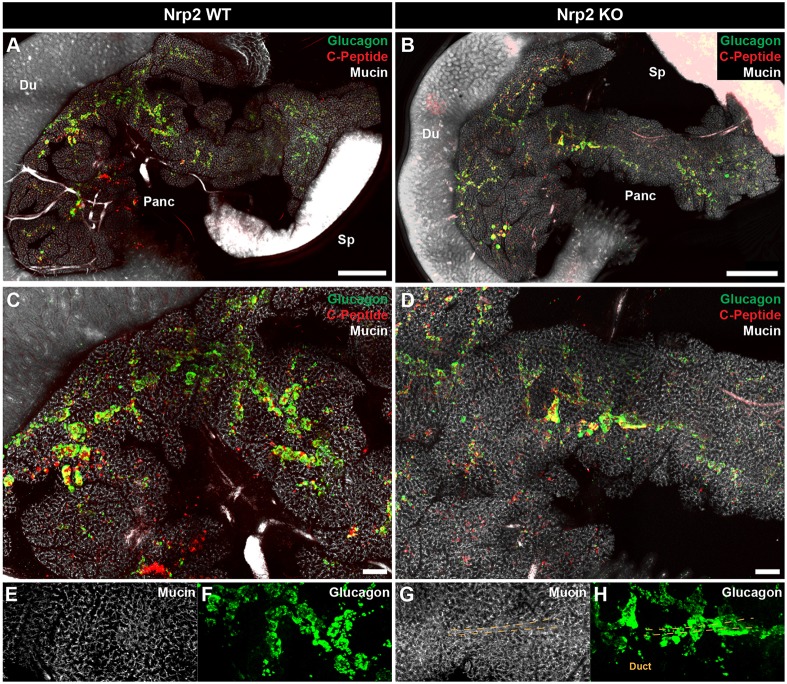
Prior studies of islet morphogenesis have been limited to reconstruction of three-dimensional islet phenotypes from two-dimensional imaging. To characterize islet developmental defects in the intact pancreas of Nrp2 mutants at P1, we performed whole-organ confocal imaging using CLARITY (Chung et al., 2013; Tomer et al., 2014). This method permits optical clearing of large tissues while preserving protein and nucleic acid localization, enabling phenotyping of intact organs with cellular resolution. After clearing the pancreas at P1, we assessed islet hormones and ductal markers by immunofluorescence in Nrp2 wild-type and knockout mice. In controls, islets formed as round clusters distributed both near to and remote from the central ductal network (Fig. 4A,C,E,F; Movie 1). In contrast, islet cells in Nrp2 knockout mice grew in streams along large central ductal structures (Fig. 4B,D,G,H; Movie 2). Thus, whole-organ imaging of intact pancreata corroborated the morphometric quantification of islet distribution we obtained with sectioned tissue, and supported a model in which Nrp2 signaling is essential for separation and dispersion of islets from their ductal origin during development.
Fig. 4.
Whole-organ imaging to assess islet morphology in Nrp2 knockouts using CLARITY. (A,B) Visualization of islet morphogenesis defects at P1 in three dimensions in the intact pancreas by confocal imaging. Du, duodenum; Sp, spleen; Panc, pancreas. (C,D) Control islets are rounded and begin to form distinct structures at P1, whereas Nrp2 knockout islet cells form continuous streams along ductal structures. (E-H) Higher magnification views of CLARITY images showing a large ductal structure in a knockout (G) surrounded by a continuous mass of endocrine cells (H, arrowheads and dashed lines indicate example duct structures surrounded by islets). Compare with corresponding regions of control pancreas (E,F). Scale bars: 1 mm in A,B; 200 μm in C,D.
Defective cell migration and deformation in endocrine cells lacking Nrp2
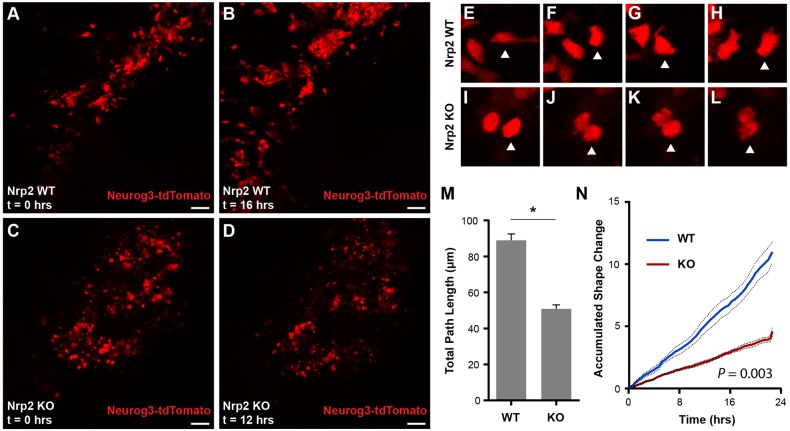
Our analysis of fixed tissues suggested that semaphorin-Nrp2 signaling is essential for migration of developing pancreatic endocrine cells. To test this hypothesis directly, we used live cell imaging to quantify movement and cell shape changes in fetal islet cells (Pauerstein et al., 2015). We used mice harboring a Neurog3-tdTomato transgene, which labels all Neurog3+ endocrine progenitors and their hormone-expressing progeny (Sugiyama et al., 2013). Time lapse confocal imaging of cultured E13.5 Nrp2+/+; Neurog3-tdTomato control organs for 24 h revealed extensive migration and deformation of developing endocrine cells, including extension of filopodia (Fig. 5A,B,E-H; Movie 3). In contrast, movement of Neurog3-tdTomato+ cells in Nrp2 mutants was limited, and these cells failed to undergo deformation (Fig. 5C,D,I-L; Movie 4). Mutant islet cells often remained rounded and near their origin throughout imaging, and many cells did not extend detectable filopodia (Fig. 5E-L; Movie 4). Quantification of cell displacement confirmed a reduction in total distance traveled by single islet cells in Nrp2 mutants (P=2×10−11; Fig. 5M, Fig. S5). Measurement of total cell shape change during imaging confirmed a significant reduction of cell deformation (P=0.003, Fig. 5N). These experiments indicate that Nrp2 is required for fetal islet cell migration and deformation, including cell biological processes such as the filopodia extension that is integral to migration, and provide a cellular mechanism linking defective Nrp2 signaling to defects in islet morphogenesis.
Fig. 5.
Loss of Nrp2 results in defective cell deformation and migration during islet development. (A,B) Frames from time-lapse confocal imaging of Neurog3-tdTomato cells in Nrp2 wild-type organ cultures. Fetal islet cells undergo deformation, migration and clustering over the 24 h imaging experiment. (C,D) Nrp2 knockout islet cells fail to undergo normal cell deformation and migration. (E-L) Time-lapse images of single-cell deformation phenotypes in Nrp2 wild-type and knockout cells. Note deformation and extension of cell processes in E,G,H. Arrowheads indicate one cell tracked over time for each genotype. (M) Quantification of total distance traveled by individual Nrp2 wild-type and knockout cells (*P=2×10−11). (N) Quantification of cell shape changes over time in Nrp2 knockout cells (P=0.003, two-tailed t-test). Scale bars: 50 μm. Data are mean±s.e.m. Data points were from 38 wild-type and 25 knockout cells, using videos from two separate experiments.
DISCUSSION
We used classical organ culture and genetics to identify mesenchyme-derived cues influencing islet development, and found that semaphorins are unrecognized chemoattractants for α and β cells. Purified Sema3a was sufficient to redirect fetal α and β cell migration toward beads, indicating that semaphorin signaling provides instructive, not merely permissive, cues to these islet cells. Spatially localized production of Sema3a or other semaphorins by peripheral mesenchymal cells could provide instructive cues to guide radial migration of Nrp2+ islet cells (Fig. 6). Consistent with this model, loss of Nrp2, a receptor for secreted semaphorins, resulted in impaired cell migration and defects in islet morphogenesis. Thus, our findings suggest that semaphorins are potent long-range chemoattractants for fetal α and β cells, defining a radial axis that controls islet morphogenesis at both unicellular and multicellular levels. To our knowledge, long-range or spatially localized guidance cues coordinating islet morphogenesis have not previously been identified. Future studies, perhaps using conditional genetics in specific cell subsets, could assess the principal semaphorins responsible for regulating islet morphogenesis. Our data raise the likelihood that multiple semaphorins, including Sema3a and Sema3d, could serve this function.
Fig. 6.
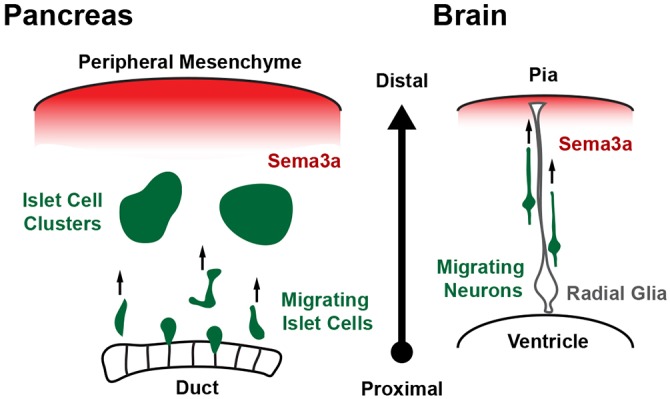
Model for semaphorin signaling in islet cell radial migration. In the developing pancreas, semaphorin 3a emanates from the peripheral mesenchyme and acts as a chemoattractant for islet cells expressing the semaphorin receptor Nrp2. This signal directs islet cell migration away from their birthplaces in the central ducts and towards the periphery, where they cluster to form islets. Sema3a plays a similar role as a guidance cue in cortical development. There, a peripheral Sema3a signal induces neuropilin- and plexin-dependent radial migration of developing neurons away from the ventricles and towards the pial surface of the cortex.
While Sema3a stimulated migration of both fetal α and β cells, this effect appeared more pronounced with α cells. We also found differences in Nrp2 expression between α and β cells: fetal α cell Nrp2 expression was durable and persisted in adult α cells, unlike in β cells, which showed only transient fetal-stage expression. To the extent that Nrp2 appears to be essential for Sema-mediated signaling in fetal islets, our findings raise the possibility that Sema-Nrp2 signaling primarily guides α cell migration and initial β cell migration. In the later stages of islet morphogenesis, it is possible that α cells then influence β cell migration through cues yet to be identified. Our findings also suggest possible functions for Nrp2 signaling in adult islet α cells. These models could be tested by conditional inactivation of Nrp2 in fetal or adult α cells, though we found that the extant conditional ‘floxed’ Nrp2f allele (Walz et al., 2002) was resistant to inactivation by Cre recombinase transgenes, including Pdx1-Cre or Ngn3-Cre. These have been used previously to inactivate other floxed alleles in the pancreas or fetal islets (Goodyer et al., 2012; Gu et al., 2002). Thus, alternative approaches, perhaps including the generation of additional Nrp2f alleles, may be required to pursue conditional genetics in the pancreas.
Semaphorin signaling through Nrp-plexin receptors can be transduced through multiple intracellular pathways that regulate cytoskeletal dynamics leading to oriented cell movement, including Rho GTPases, the Ras pathway or via Cdk5 signaling (Ahmed and Eickholt, 2007; Eickholt et al., 2002; Takahashi et al., 1999). Our preliminary data support a role for Nrp2 signaling through Cdk5 and Rho intermediaries in semaphorin-induced migration of nascent islet cells (P.T.P., K.T. and S.K.K., unpublished). In addition to Sema3a signaling through plexin receptors to cytoskeleton effector proteins, it is also possible that Sema3a signaling leads to an interaction between Nrp2 and integrins to affect cell migration. Nrp2 interactions with integrins at focal adhesions within the same cell (cis) permit integrin binding to laminin in the extracellular matrix, whereas Nrp2 binding to integrins on endothelial cells (trans) allows cancer cell intravasation and extravasation; both of these processes lead to an increase in cancer cell migration or metastasis and poor prognosis (Goel et al., 2012; Cao et al., 2013). Considering that many cancer mechanisms have been adapted from normal developmental events, it is conceivable that such a mechanism might also occur in normal islet morphogenesis.
Distribution of the islets of Langerhans throughout the exocrine tissue is a cardinal pancreatic feature in most vertebrates – including fish, birds, snakes, rodents and primates – suggesting selective forces have maintained this anatomy (Conlon et al., 1988; Moscona, 1990; Steiner et al., 2010). Thus, islet morphogenesis and dispersion is likely controlled by multiple conserved mechanisms. Initially, islet progenitor cells within the fetal ductal epithelium delaminate and, while differentiating, migrate into nascent islet clusters. Multicellular clusters enlarge, reflecting continued cell aggregation and proliferation, and at later fetal and postnatal stages islet cells rearrange to form morphologically mature islets dispersed throughout the exocrine pancreas (Benitez et al., 2012). Our analysis of Nrp2 mutant mice suggests that the initial differentiation, delamination and clustering of islet cells do not require Sema-Nrp signaling. However, in Nrp2 mutants, islet cells failed to establish normal dispersed islet morphology, leading to abnormally large islet cell aggregates surrounding and connected to major ducts. We postulate that normal islet dispersion could reflect the combination of at least two distinct processes: (1) active directional islet cell migration mediated by a combination of long- and short-range cues, both attractive and repulsive; and (2) growth of intervening non-islet tissues, most abundantly acinar and ductal cells. Other signals are likely involved in islet dispersion: we identified multiple candidate signaling pathways with effects on islet cell development in our screen. However, we focused on semaphorin signaling because of the observed phenotypes in our assays and the availability of reagents to perturb the semaphorin pathway in the pancreas. As pancreatic growth was not detectably impaired in Nrp2 mutants at birth, tissue growth is likely not sufficient to disperse islets during embryogenesis. In an evolutionary context, the arrangement of islet cells contiguous with their originating epithelium observed in Nrp2 mutants is reminiscent of findings from jawless fish, where cells expressing pancreatic hormones such as insulin, pancreatic polypeptide and glucagon are found adjacent to or within homologous foregut intestinal epithelium (Conlon et al., 1988; Falkmer, 1979).
The sharing of genetic or signaling modules used to build morphologically or phylogenetically disparate features has been called ‘deep homology’ (Shubin et al., 1997, 2009). Unexpectedly, we found that key elements of a radially oriented signaling pathway regulating cortical lamination in central nervous system development are also deployed for dispersion of islets during pancreas development. During mammalian brain development, a stereotyped laminar organization of neurons in the cortex is essential for cortical function. In mice, semaphorins at the distal pial surface signal to postmitotic neurons derived from proximal ventricular zone neural stem cells, directing radial migration toward the pial surface along radial glial cells as cortical lamination proceeds (Chen et al., 2008). In that context, plexin receptors signal through Rho GTPases to direct cell migration towards outer cortical layers (Azzarelli et al., 2014; Pacary et al., 2013). Signaling via Cdk5 also controls multiple aspects of neuronal radial migration and cortical lamination (Chae et al., 1997; Su and Tsai, 2011). Ectopic Sema3a or loss of Nrp1 is sufficient to disrupt development of this layering. Thus, cortical lamination and pancreatic islet morphogenesis appear to share strikingly similar signaling molecules, cellular processes and developmental axis arrangement (Fig. 6). Sema3a signaling in brain cortical lamination may extend up to several hundred micrometers, comparable with or longer than the distances involved in mouse islet development. Our findings also support prior work that revealed striking similarities between mammalian islet cells and neurons (Ohta et al., 2011; Rulifson et al., 2002; Van Noorden and Falkmer, 1980). As in nervous system development, additional signals conveying chemoattractive, chemorepulsive or ‘stop’ cues might influence islet development. We speculate that an undetected radial or laminar patterning of islets may be linked to specialized functions, responsiveness or control. Consistent with this notion is the observation that human islet inflammation, destruction or preservation in type 1 diabetes mellitus may occur in a heterogeneous pattern (Gaglia et al., 2015; In't Veld, 2014; Poudel et al., 2015), and that autonomic nerves link groups of islets (Reinert et al., 2014; P.T.P., B.H., K.D. and S.K.K., unpublished).
Discovery of a long-range islet chemoattractant could also influence regenerative approaches to islet reconstitution. In hematopoietic stem cell transplantation, chemokine-guided migration of stem cells to developmental niches illustrates the therapeutic applications of cellular homing (Copelan, 2006). We speculate that studies of islet guidance cues might help establish similar homing and engraftment paradigms for islet cells or their progenitors.
MATERIALS AND METHODS
Animals
All animal experiments were conducted in accordance with Stanford University IACUC guidelines. Mouse Insulin1 promoter-GFP transgenic mice were purchased from Jackson Laboratories (Hara et al., 2003) and maintained on a CD1 genetic background. Nrp1 floxed (Gu et al., 2003) and Nrp2 knockout mice (Giger et al., 2000) were obtained with permission from Dr David Ginty (Harvard Medical School, Boston, MA, USA) and maintained on a Black6 background. Neurog3-tdTomato transgenic mice have been previously reported and were maintained on a CD1 background (Sugiyama et al., 2013), as were Sema3dGFP-Cre knock-in mice (Katz et al., 2012). Neurog3gfp/+ mice were obtained from Klaus Kaestner (University of Pennsylvania, USA) and maintained on a CD1 background (Lee et al., 2002). Wnt1-Cre (Danielian et al., 1998; Lewis et al., 2013) and Rosa-mTmG (Muzumdar et al., 2007) mice were obtained from the Jackson Laboratories and maintained on a mixed Black6 and CD1 background. Transgenic Ngn3-Cre and Pdx1-Cre mice have been previously described (Gu et al., 2002). Floxed Nrp2 mice (Walz et al., 2002) were obtained from the Kolodkin lab (Johns Hopkins School of Medicine and HHMI, MD, USA) and maintained by the Epstein and Kim labs. Experiments and morphometry analysis were performed on mice at embryonic day (E) 13.5, E15.5, postnatal day 1 and 6 months; both male and female mice were used in all experiments.
Organ culture and live imaging
For organ culture experiments, fetal pancreatic rudiments were dissected from E13.5 mouse embryos, embedded in type 1 collagen gel (EMD Millipore) and cultured in DMEM/F12 supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin for experiments not involving beads (Gittes et al., 1996; Puri and Hebrok, 2007). The FBS concentration was reduced to 0.5% for bead assays in the screen. Medium was changed every other day for experiments not involving beads. For bead assays, one half of the media volume was refreshed daily for the duration of experiments. Organs were fixed in 4% PFA and then processed for whole-mount immunofluorescence or for cryosectioning.
For live-imaging experiments, E13.5 organs were dissected and visually assessed for transgene presence. For Nrp2 knockout experiments, rapid genotyping was performed using the Phire Tissue Direct kit (Thermo Fisher). Samples were mounted in type 1 collagen gel (EMD Millipore) in glass-bottomed dishes (MatTek) and cultured in organ culture media without Phenol Red (described above) supplemented with 1% insulin-transferrin-selenium. Images were acquired using a Leica SP8 inverted confocal system equipped with a white light laser and an environmental control chamber using a 20× water immersion objective. For detection of tdTomato, samples were excited at 561 nm. Images were acquired at 20 min intervals for all time-lapse experiments. Data were saved in LIF format and analyzed using Volocity image analysis software (Perkin Elmer) or ImageJ. Quantification of cell shape changes have been described elsewhere (Pauerstein et al., 2015). Cell displacement and velocity measurements were calculated using the MTrackJ plugin in ImageJ (Meijering et al., 2012).
Bead screen
Affi-Gel Blue beads, 100-200 mesh (Bio Rad 153-7302), were incubated in 100 ng/ml growth factor overnight at 4°C. Beads were washed in sterile PBS before implanting into mouse fetal organ culture in three locations: one in the ventral pancreas, one in the proximal region of the dorsal pancreas and one in the distal region of the dorsal pancreas. For screening, bright-field and GFP images were acquired at days 1 and 5 using a Leica MZ16FA stereomicroscope, with a constant exposure time and total magnification of 50× maintained for all assays. Images were opened in ImageJ (Schneider et al., 2012) and Ins1-GFP intensity as a function of radius was calculated using the ‘Radial Profile’ plug-in. Images were converted to grayscale, the center of the bead was defined by the user and intensity was calculated from r=0 pixels to r=250 pixels (325 μm) using the ‘Radial Profile’ tool. Only beads in the proximal dorsal pancreas were assessed for calculating shift values, because of the favorable optical properties of the tissue in that region. Results tables relating radius and GFP intensity were exported to custom templates in Microsoft Excel, in which the formula:
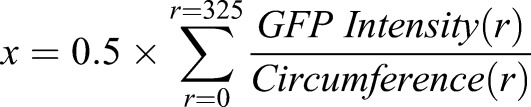 |
was used to calculate mean radial positions corresponding to the weighted centers of intensity plots. GFP Intensity (r) refers to the sum of pixel intensity values along the circle defined by the radius r from the center of the bead, and Circumference (r) refers to the circumference of that circle. Weighted centers of intensity at day 0 were subtracted from values at day 5 for each individual pancreas to generate ‘shift’ values, which describe changes in overall β cell distribution around beads: Shift=xday 5−xday 0. Negative shift values indicate an attractive effect on β cells, whereas positive shift values indicate a repulsive effect. Shift values were calculated for three to seven organs per candidate signal, averaged and compared with PBS control bead assays using a two-tailed Student's t-test. P values less than 0.05 were considered significant. Growth factors used are indicated in Table S1. Chemical inhibitors used are indicated in Table S2.
Immunostaining
Tissue was fixed in 4% PFA overnight and processed for cryosectioning. Samples were blocked in 1% BSA and 0.2% nonfat evaporated milk supplemented with 0.5% Triton X-100 and 1% DMSO, and antibody incubations were performed in blocking buffer. AlexaFluor- and Dylight-conjugated secondary antibodies (Life Technologies and Jackson Immunoresearch) were used for detection. Antibodies used are indicated in Table S3.
For all morphometric analyses, images representing entire pancreatic sections were acquired using a Zeiss AxioM1 microscope. At least 12 sections of 10 μm thickness and separated by at least 120 μm were analyzed for each organ. For quantification of α and β cell areas, tissue was immunostained for E-Cadherin and appropriate hormone markers. E-Cadherin-positive areas were manually traced in ImageJ, and images were thresholded to calculate hormone-positive areas using the ‘Analyze Particles’ function. Total hormone+ areas were divided by total E-Cadherin+ areas for each biological sample. For duct-islet distance measurements, tissue was labeled using DBA lectin and antibodies to ChgA. Distances from ChgA+ islet edges to nearest DBA lectin+ structure were measured using the ‘Line’ function in ImageJ. All islet clusters larger than 4 cells were measured in all sections analyzed for each pancreas. For assessment of duct contact with islets, ducts cut in cross-section and adjacent to islets were visually identified. The ImageJ ‘Angle’ tool was used to measure the angle defined by the center of the duct and the two edges of islet contact with the basal surface of the duct. Data were exported to Microsoft Excel and Graphpad Prism for analysis. Distance or angle measurements were averaged for each independent sample, and biological groups were compared using statistical methods described below. Measurements were performed by individuals blinded to the genotypes of samples.
CLARITY methods
Whole organ analysis of pancreatic islet morphology was performed using CLARITY (Chung et al., 2013; Hsueh et al., 2017) with several modifications. Tissue was dissected in ice-cold PBS and immediately incubated in hydrogel monomer solution for 3-5 days at 4°C. The hydrogel monomer solution is 4% PFA, 4% acrylamide, 0.25% VA-044 initiator and 0.05% saponin in PBS. Bis-acrylamide, included in the original formulation (Chung et al., 2013), was omitted to prevent polymerization of hydrogel outside the tissue. Tissue was cleared passively for 5-14 days by incubating in clearing buffer (4% SDS, 200 mM boric acid at pH 8.5) at 55°C (Tomer et al., 2014). After clearing, tissue was washed in PBS and immunostained using standard protocols, with antibody incubations extended to 3-5 days to permit penetration of thick samples. Samples were mounted in FocusClear (CelExplorer Labs) and imaged using an Olympus confocal microscope equipped with 5× air and 10× water immersion objectives. Tiled z-stack datasets were stitched into a single z-stack using Fiji (Schindelin et al., 2012) and were visualized using Volocity image analysis software. 3D reconstruction videos were created using Arivis Vision4D software.
FACS and gene expression measurement
Pancreatic rudiments were dissected from E15.5 embryos and processed as previously described (Sugiyama et al., 2007), with the exception that organs were dissociated using Accutase (eBioscience). Single-cell suspensions were stained using antibodies described in Table S1 and were sorted using a BD FACS Aria II. Red blood cells were depleted from the cell fractions using RBC lysis buffer (BioLegend). Cells were collected in PBS supplemented with 2% BSA and 10 mM EGTA. RNA was purified using the PicoPure RNA extraction system (Life Technologies), cDNA was synthesized using Superscript III reverse transcriptase (Life Technologies), and gene expression was measured using qPCR with Taqman probes and an ABI7500 qPCR system (Applied Biosystems).
For analysis of plexin gene expression in FACS-purified cell populations, E15.5 Neurog3gfp/+ pancreas was obtained and processed for FACS as above, with fractions defined as follows: GFP+CD133lowEpCAMlow/negCD31− cells were considered to be ‘endocrine’, EpCAM+GFP− cells were ‘EpCAM’, CD31+GFP−EpCAM− cells were ‘CD31’ and EpCAM−GFP−CD31− cells were considered ‘Negative’ and represent a mixed population of cells not encompassed by the other cell fractions (Benitez et al., 2014; Sugiyama et al., 2007). CD45+ white blood cells were excluded from all fractions, and red blood cells were depleted as described above.
Gene expression analysis using quantitative RT-PCR was performed using the following Taqman probes (Life Technologies): Sema3a, Mm00436469; Sema3d, Mm01224783; Pecam, Mm01242584; S100a4, Mm00803372; vimentin, Mm01333430; Acta2, Mm00725412; Syp, Mm00436850; Sox9, Mm00448840; Ins2, Mm00731595; Plxna3, Mm00501170; Plxnb1, Mm00555359; and Plxnb2, Mm00507118.
To assess plexin family gene expression by RT-PCR, RNA was isolated from microdissected whole E15.5 wild-type mouse pancreas (CD1 background) using the PicoPure RNA Isolation Kit (Life Technologies). cDNA was synthesized using Superscript III reverse transcriptase, and gene expression was assessed by PCR using BioMix Red (Bioline) and the primer sets indicated in Table S4.
In situ hybridization
RNA in situ hybridization was performed using the RNAscope system (Advanced Cell Diagnostics, ACD). Probes for mouse Sema3a (ACD 412961) and Polr2a (ACD 312471) were purchased from ACD, as were RNAscope 2.0 (Brown reagents). For fluorescence detection, tyramide signal amplification reagents (Life Technologies) were used in place of DAB reagents. In situ hybridizations were performed on freshly sectioned cryoembedded tissue samples at stages indicated in the text.
Human tissue samples
Experiments involving human tissue samples were approved by the Stanford University School of Medicine Institutional Review Board. Human pancreatic tissue between gestational days (GD) 75 and 110 was obtained through the Birth Defects Research Laboratory, University of Washington (Seattle, WA, USA). All donors provided informed consent.
Statistical analysis
Data are presented as mean±s.e.m. unless otherwise indicated. Student's t-test was used for statistical comparisons unless otherwise noted. P values less than 0.05 were considered significant. Statistical analysis was performed using Graphpad Prism and Microsoft Excel software.
Supplementary Material
Acknowledgements
We thank members of the Kim Lab for helpful discussion and advice, Dr Jon Mulholland and Kitty Lee of the Stanford Cell Sciences Imaging Facility for assistance with confocal microscopy, and Drs P. Lwigale (Rice University), M. Scott (Stanford University) and R. Nusse (Stanford University) for sharing reagents.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: P.T.P., S.K.K.; Methodology: P.T.P., K.T., K.B.W., K.M.P., B.H., J.A.E., S.K.K.; Validation: P.T.P., K.T., K.M.P., B.H., S.K.K.; Formal analysis: P.T.P., K.T., K.B.W., K.M.P., B.H., X.G.; Investigation: P.T.P., K.T., K.B.W., K.M.P., B.H., H.E.A., X.G., H.A., K.D., S.K.K.; Resources: H.A., K.D., J.A.E., S.K.K.; Data curation: P.T.P.; Writing - original draft: P.T.P., S.K.K.; Writing - review & editing: P.T.P., K.T., K.B.W., K.M.P., B.H., H.A., K.D., J.A.E., S.K.K.; Visualization: P.T.P., K.T., B.H.; Supervision: K.D., J.A.E., S.K.K.; Project administration: S.K.K.; Funding acquisition: S.K.K.
Funding
P.T.P. was supported by the Howard Hughes Medical Institute Medical Fellows program, the Stanford Medical Scientist Training Program and by the National Institutes of Health (F30DK102301). K.T. was supported by a National Science Foundation graduate research fellowship (DGE-114747). H.E.A. was supported by a Juvenile Diabetes Research Foundation postdoctoral fellowship. J.A.E. is supported by the National Institutes of Health (HL118768). Work in the Kim lab was supported by the Howard Hughes Medical Institute, Juvenile Diabetes Research Foundation, the Leona M. and Harry B. Helmsley Charitable Trust, and the H. L. Snyder Medical Foundation. Deposited in PMC for release after 6 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.148684.supplemental
References
- Acloque H., Adams M. S., Fishwick K., Bronner-Fraser M. and Nieto M. A. (2009). Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J. Clin. Invest. 119, 1438-1449. 10.1172/JCI38019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afelik S., Qu X., Hasrouni E., Bukys M. A., Deering T., Nieuwoudt S., Rogers W., MacDonald R. J. and Jensen J. (2012). Notch-mediated patterning and cell fate allocation of pancreatic progenitor cells. Development 139, 1744-1753. 10.1242/dev.075804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A. and Eickholt B. J. (2007). Intracellular kinases in semaphorin signaling. Adv. Exp. Med. Biol. 600, 24-37. 10.1007/978-0-387-70956-7_3 [DOI] [PubMed] [Google Scholar]
- Anderson K. R., Singer R. A., Balderes D. A., Hernandez-Lagunas L., Johnson C. W., Artinger K. B. and Sussel L. (2011). The L6 domain tetraspanin Tm4sf4 regulates endocrine pancreas differentiation and directed cell migration. Development 138, 3213-3224. 10.1242/dev.058693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelqvist A., Li H., Sommer L., Beatus P., Anderson D. J., Honjo T., Hrabe de Angelis M., Lendahl U. and Edlund H. (1999). Notch signalling controls pancreatic cell differentiation. Nature 400, 877-881. 10.1038/23716 [DOI] [PubMed] [Google Scholar]
- Azzarelli R., Pacary E., Garg R., Garcez P., van den Berg D., Riou P., Ridley A. J., Friedel R. H., Parsons M. and Guillemot F. (2014). An antagonistic interaction between PlexinB2 and Rnd3 controls RhoA activity and cortical neuron migration. Nat. Commun. 5, 3405 10.1038/ncomms4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez C. M., Goodyer W. R. and Kim S. K. (2012). Deconstructing pancreas developmental biology. Cold Spring Harb. Perspect. Biol. 4, a012401 10.1101/cshperspect.a012401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez C. M., Qu K., Sugiyama T., Pauerstein P. T., Liu Y., Tsai J., Gu X., Ghodasara A., Arda H. E., Zhang J. et al. (2014). An integrated cell purification and genomics strategy reveals multiple regulators of pancreas development. PLoS Genet. 10, e1004645 10.1371/journal.pgen.1004645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan A., Itoh N., Kato S., Thiery J. P., Czernichow P., Bellusci S. and Scharfmann R. (2001). Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development 128, 5109-5117. [DOI] [PubMed] [Google Scholar]
- Blum B., Roose A. N., Barrandon O., Maehr R., Arvanites A. C., Davidow L. S., Davis J. C., Peterson Q. P., Rubin L. L. and Melton D. A. (2014). Reversal of β cell de-differentiation by a small molecule inhibitor of the TGFβ pathway. eLife 3, e02809 10.7554/eLife.02809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M., Shostak A., Shiota M., Wiebe P. O., Poffenberger G., Kantz J., Chen Z., Carr C., Jerome W. G., Chen J. et al. (2006). Pancreatic islet production of vascular endothelial growth Factor-A is essential for islet vascularization, revascularization, and function. Diabetes 55, 2974-2985. 10.2337/db06-0690 [DOI] [PubMed] [Google Scholar]
- Cai Q., Brissova M., Reinert R. B., Cheng Pan F., Brahmachary P., Jeansson M., Shostak A., Radhika A., Poffenberger G., Quaggin S. E. et al. (2012). Enhanced expression of VEGF-A in β cells increases endothelial cell number but impairs islet morphogenesis and β cell proliferation. Dev. Biol. 367, 40-54. 10.1016/j.ydbio.2012.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Hoeppner L. H., Bach S. E. G., Guo Y., Wang E., Wu J., Cowley M. J., Chang D. K., Waddell N. et al. (2013). Neuropilin-2 promotes extravasation and metastasis by interacting with endothelial α5 integrin. Cancer Res. 73, 4579-4590. 10.1158/0008-5472.CAN-13-0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae T., Kwon Y. T., Bronson R., Dikkes P., Li E. and Tsai L.-H. (1997). Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron 18, 29-42. 10.1016/S0896-6273(01)80044-1 [DOI] [PubMed] [Google Scholar]
- Chen H., Chédotal A., He Z., Goodman C. S. and Tessier-Lavigne M. (1997). Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 19, 547-559. 10.1016/S0896-6273(00)80371-2 [DOI] [PubMed] [Google Scholar]
- Chen G., Sima J., Jin M., Wang K.-Y., Xue X.-J., Zheng W., Ding Y.-Q. and Yuan X.-B. (2008). Semaphorin-3A guides radial migration of cortical neurons during development. Nat. Neurosci. 11, 36-44. 10.1038/nn2018 [DOI] [PubMed] [Google Scholar]
- Chen H., Gu X., Liu Y., Wang J., Wirt S. E., Bottino R., Schorle H., Sage J. and Kim S. K. (2011). PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature 478, 349-355. 10.1038/nature10502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K., Wallace J., Kim S.-Y., Kalyanasundaram S., Andalman A. S., Davidson T. J., Mirzabekov J. J., Zalocusky K. A., Mattis J., Denisin A. K. et al. (2013). Structural and molecular interrogation of intact biological systems. Nature 497, 332-337. 10.1038/nature12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O. and Dor Y. (2012). Vascular instruction of pancreas development. Development 139, 2833-2843. 10.1242/dev.065953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen T., Herzog Y., Brodzky A., Greenson J. K., Eldar S., Gluzman-Poltorak Z., Neufeld G. and Resnick M. B. (2002). Neuropilin-2 is a novel marker expressed in pancreatic islet cells and endocrine pancreatic tumours. J. Pathol. 198, 77-82. 10.1002/path.1179 [DOI] [PubMed] [Google Scholar]
- Conlon J. M., Reinecke M., Thorndyke M. C. and Falkmer S. (1988). Insulin and other islet hormones (somatostatin, glucagon and PP) in the neuroendocrine system of some lower vertebrates and that of invertebrates--a minireview. Horm. Metab. Res. Horm. Stoffwechselforschung Horm. Métabolisme 20, 406-410. 10.1055/s-2007-1010849 [DOI] [PubMed] [Google Scholar]
- Copelan E. A. (2006). Hematopoietic stem-cell transplantation. N. Engl. J. Med. 354, 1813-1826. 10.1056/NEJMra052638 [DOI] [PubMed] [Google Scholar]
- Cras-Méneur C., Li L., Kopan R. and Permutt M. A. (2009). Presenilins, Notch dose control the fate of pancreatic endocrine progenitors during a narrow developmental window. Genes Dev. 23, 2088-2101. 10.1101/gad.1800209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian P. S., Muccino D., Rowitch D. H., Michael S. K. and McMahon A. P. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. CB 8, 1323-1326. 10.1016/S0960-9822(07)00562-3 [DOI] [PubMed] [Google Scholar]
- Degenhardt K., Singh M. K., Aghajanian H., Massera D., Wang Q., Li J., Li L., Choi C., Yzaguirre A. D., Francey L. J. et al. (2013). Semaphorin 3d signaling defects are associated with anomalous pulmonary venous connections. Nat. Med. 19, 760-765. 10.1038/nm.3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickholt B. J., Walsh F. S. and Doherty P. (2002). An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. J. Cell Biol. 157, 211-217. 10.1083/jcb.200201098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J. A., Aghajanian H. and Singh M. K. (2015). Semaphorin signaling in cardiovascular development. Cell Metab. 21, 163-173. 10.1016/j.cmet.2014.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esni F., Ghosh B., Biankin A. V., Lin J. W., Albert M. A., Yu X., MacDonald R. J., Civin C. I., Real F. X., Pack M. A. et al. (2004). Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development 131, 4213-4224. 10.1242/dev.01280 [DOI] [PubMed] [Google Scholar]
- Falkmer S. (1979). Immunocytochemical studies of the evolution of islet hormones. J. Histochem. Cytochem. Off. J. Histochem. Soc. 27, 1281-1282. 10.1177/27.9.383830 [DOI] [PubMed] [Google Scholar]
- Fukuda T., Takeda S., Xu R., Ochi H., Sunamura S., Sato T., Shibata S., Yoshida Y., Gu Z., Kimura A. et al. (2013). Sema3A regulates bone-mass accrual through sensory innervations. Nature 497, 490-493. 10.1038/nature12115 [DOI] [PubMed] [Google Scholar]
- Gaglia J. L., Harisinghani M., Aganj I., Wojtkiewicz G. R., Hedgire S., Benoist C., Mathis D. and Weissleder R. (2015). Noninvasive mapping of pancreatic inflammation in recent-onset type-1 diabetes patients. Proc. Natl. Acad. Sci. USA 112, 2139-2144. 10.1073/pnas.1424993112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger R. J., Cloutier J.-F., Sahay A., Prinjha R. K., Levengood D. V., Moore S. E., Pickering S., Simmons D., Rastan S., Walsh F. S. et al. (2000). Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron 25, 29-41. 10.1016/S0896-6273(00)80869-7 [DOI] [PubMed] [Google Scholar]
- Gittes G. K. (2009). Developmental biology of the pancreas: a comprehensive review. Dev. Biol. 326, 4-35. 10.1016/j.ydbio.2008.10.024 [DOI] [PubMed] [Google Scholar]
- Gittes G. K., Galante P. E., Hanahan D., Rutter W. J. and Debase H. T. (1996). Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development 122, 439-447. [DOI] [PubMed] [Google Scholar]
- Goel H. L., Pursell B., Standley C., Fogarty K. and Mercurio A. (2012). Neuropilin-2 regulates α6β1 integrin in the formation of focal adhesions and signaling. J. Cell Sci. 125, 497-506. 10.1242/jcs.094433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golosow N. and Grobstein C. (1962). Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev. Biol. 4, 242-255. 10.1016/0012-1606(62)90042-8 [DOI] [PubMed] [Google Scholar]
- Goodyer W. R., Gu X., Liu Y., Bottino R., Crabtree G. R. and Kim S. K. (2012). Neonatal β cell development in mice and humans is regulated by calcineurin/NFAT. Dev. Cell 23, 21-34. 10.1016/j.devcel.2012.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzi M., Kim Y. H., Katsumoto K., Johansson K. and Grapin-Botton A. (2011). Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 240, 589-604. 10.1002/dvdy.22544 [DOI] [PubMed] [Google Scholar]
- Greiner T. U., Kesavan G., Ståhlberg A. and Semb H. (2009). Rac1 regulates pancreatic islet morphogenesis. BMC Dev. Biol. 9, 2 10.1186/1471-213X-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G., Dubauskaite J. and Melton D. A. (2002). Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447-2457. [DOI] [PubMed] [Google Scholar]
- Gu C., Rodriguez E. R., Reimert D. V., Shu T., Fritzsch B., Richards L. J., Kolodkin A. L. and Ginty D. D. (2003). Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5, 45-57. 10.1016/S1534-5807(03)00169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Landsman L., Li N. and Hebrok M. (2013). Factors expressed by murine embryonic pancreatic mesenchyme enhance generation of insulin-producing cells from hESCs. Diabetes 62, 1581-1592. 10.2337/db12-0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Wang X., Kawamura T., Bindokas V. P., Dizon R. F., Alcoser S. Y., Magnuson M. A. and Bell G. I. (2003). Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 284, E177-E183. 10.1152/ajpendo.00321.2002 [DOI] [PubMed] [Google Scholar]
- Hsueh B., Burns V. M., Pauerstein P., Holzem K., Ye L., Engberg K., Wang A.-C., Gu X., Chakravarthy H., Arda H. E. et al. (2017). Pathways to clinical CLARITY: volumetric analysis of irregular, soft, and heterogeneous tissues in development and disease. Sci. Rep. 7, 5899 10.1038/s41598-017-05614-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M., Kanazawa H., Kimura K., Hattori F., Ieda Y., Taniguchi M., Lee J.-K., Matsumura K., Tomita Y., Miyoshi S. et al. (2007). Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat. Med. 13, 604-612. 10.1038/nm1570 [DOI] [PubMed] [Google Scholar]
- In't Veld P. (2014). Insulitis in human type 1 diabetes: a comparison between patients and animal models. Semin. Immunopathol. 36, 569-579. 10.1007/s00281-014-0438-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz T. C., Singh M. K., Degenhardt K., Rivera-Feliciano J., Johnson R. L., Epstein J. A. and Tabin C. J. (2012). Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev. Cell 22, 639-650. 10.1016/j.devcel.2012.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavan G., Lieven O., Mamidi A., Öhlin Z. L., Johansson J. K., Li W.-C., Lommel S., Greiner T. U. and Semb H. (2014). Cdc42/N-WASP signaling links actin dynamics to pancreatic β cell delamination and differentiation. Development 141, 685-696. 10.1242/dev.100297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K. and Hebrok M. (2001). Intercellular signals regulating pancreas development and function. Genes Dev. 15, 111-127. 10.1101/gad.859401 [DOI] [PubMed] [Google Scholar]
- Kolodkin A. L., Matthes D. J. and Goodman C. S. (1993). The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75, 1389-1399. 10.1016/0092-8674(93)90625-Z [DOI] [PubMed] [Google Scholar]
- Kolodkin A. L., Levengood D. V., Rowe E. G., Tai Y.-T., Giger R. J. and Ginty D. D. (1997). Neuropilin is a semaphorin III receptor. Cell 90, 753-762. 10.1016/S0092-8674(00)80535-8 [DOI] [PubMed] [Google Scholar]
- Konstantinova I., Nikolova G., Ohara-Imaizumi M., Meda P., Kucěra T., Zarbalis K., Wurst W., Nagamatsu S. and Lammert E. (2007). EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 129, 359-370. 10.1016/j.cell.2007.02.044 [DOI] [PubMed] [Google Scholar]
- Lammert E., Cleaver O. and Melton D. (2001). Induction of pancreatic differentiation by signals from blood vessels. Science 294, 564-567. 10.1126/science.1064344 [DOI] [PubMed] [Google Scholar]
- Lammert E., Gu G., McLaughlin M., Brown D., Brekken R., Murtaugh L. C., Gerber H.-P., Ferrara N. and Melton D. A. (2003). Role of VEGF-A in vascularization of pancreatic islets. Curr. Biol. 13, 1070-1074. 10.1016/S0960-9822(03)00378-6 [DOI] [PubMed] [Google Scholar]
- Landsman L., Nijagal A., Whitchurch T. J., Vanderlaan R. L., Zimmer W. E., Mackenzie T. C. and Hebrok M. (2011). Pancreatic mesenchyme regulates epithelial organogenesis throughout development. PLoS Biol. 9, e1001143 10.1371/journal.pbio.1001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S., Perreault N., Brestelli J. E. and Kaestner K. H. (2002). Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 16, 1488-1497. 10.1101/gad.985002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. E., Vasudevan H. N., O'Neill A. K., Soriano P. and Bush J. O. (2013). The widely used Wnt1-Cre transgene causes developmental phenotypes by ectopic activation of Wnt signaling. Dev. Biol. 379, 229-234. 10.1016/j.ydbio.2013.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijering E., Dzyubachyk O. and Smal I. (2012). Methods for cell and particle tracking. Methods Enzymol. 504, 183-200. 10.1016/B978-0-12-391857-4.00009-4 [DOI] [PubMed] [Google Scholar]
- Metzger D. E., Gasperowicz M., Otto F., Cross J. C., Gradwohl G. and Zaret K. S. (2012). The transcriptional co-repressor Grg3/Tle3 promotes pancreatic endocrine progenitor delamination and β-cell differentiation. Development 139, 1447-1456. 10.1242/dev.072892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen P. J., Huotari M., Koivisto T., Ustinov J., Palgi J., Rasilainen S., Lehtonen E., Keski-Oja J. and Otonkoski T. (2000). Impaired migration and delayed differentiation of pancreatic islet cells in mice lacking EGF-receptors. Development 127, 2617-2627. [DOI] [PubMed] [Google Scholar]
- Miralles F., Battelino T., Czernichow P. and Scharfmann R. (1998). TGF-beta plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. J. Cell Biol. 143, 827-836. 10.1083/jcb.143.3.827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. A. (1990). Anatomy of the pancreas and Langerhans islets in snakes and lizards. Anat. Rec. 227, 232-244. 10.1002/ar.1092270212 [DOI] [PubMed] [Google Scholar]
- Muñoz-Bravo J. L., Hidalgo-Figueroa M., Pascual A., López-Barneo J., Leal-Cerro A. and Cano D. A. (2013). GDNF is required for neural colonization of the pancreas. Development 140, 3669-3679. 10.1242/dev.091256 [DOI] [PubMed] [Google Scholar]
- Murtaugh L. C., Stanger B. Z., Kwan K. M. and Melton D. A. (2003). Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA 100, 14920-14925. 10.1073/pnas.2436557100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M. D., Tasic B., Miyamichi K., Li L. and Luo L. (2007). A global double-fluorescent Cre reporter mouse. Genes. N. Y. N 2000 45, 593-605. 10.1002/dvg.20335 [DOI] [PubMed] [Google Scholar]
- Ohta Y., Kosaka Y., Kishimoto N., Wang J., Smith S. B., Honig G., Kim H., Gasa R. M., Neubauer N., Liou A. et al. (2011). Convergence of the insulin and serotonin programs in the pancreatic β-cell. Diabetes 60, 3208-3216. 10.2337/db10-1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacary E., Azzarelli R. and Guillemot F. (2013). Rnd3 coordinates early steps of cortical neurogenesis through actin-dependent and -independent mechanisms. Nat. Commun. 4, 1635 10.1038/ncomms2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca F. W., Millman J. R., Gürtler M., Segel M., Van Dervort A., Ryu J. H., Peterson Q. P., Greiner D. and Melton D. A. (2014). Generation of functional human pancreatic β cells in vitro. Cell 159, 428-439. 10.1016/j.cell.2014.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauerstein P. T., Sugiyama T., Stanley S. E., McLean G. W., Wang J., Martín M. G. and Kim S. K. (2015). Dissecting human gene functions regulating islet development with targeted gene transduction. Diabetes 64, 3037-3049. 10.2337/db15-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F., Giger R. J., Ginty D. D., Kolodkin A. L. and Ghosh A. (1998). Patterning of cortical efferent projections by semaphorin-neuropilin interactions. Science 282, 1904-1906. 10.1126/science.282.5395.1904 [DOI] [PubMed] [Google Scholar]
- Poudel A., Savari O., Striegel D. A., Periwal V., Taxy J., Millis J. M., Witkowski P., Atkinson M. A. and Hara M. (2015). Beta-cell destruction and preservation in childhood and adult onset type 1 diabetes. Endocrine 49, 693-702. 10.1007/s12020-015-0534-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S. and Hebrok M. (2007). Dynamics of embryonic pancreas development using real-time imaging. Dev. Biol. 306, 82-93. 10.1016/j.ydbio.2007.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S. and Hebrok M. (2010). Cellular plasticity within the pancreas--lessons learned from development. Dev. Cell 18, 342-356. 10.1016/j.devcel.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert R. B., Brissova M., Shostak A., Pan F. C., Poffenberger G., Cai Q., Hundemer G. L., Kantz J., Thompson C. S., Dai C. et al. (2013). Vascular endothelial growth factor-a and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes 62, 4154-4164. 10.2337/db13-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert R. B., Cai Q., Hong J.-Y., Plank J. L., Aamodt K., Prasad N., Aramandla R., Dai C., Levy S. E., Pozzi A. et al. (2014). Vascular endothelial growth factor coordinates islet innervation via vascular scaffolding. Development 141, 1480-1491. 10.1242/dev.098657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania A., Bruin J. E., Arora P., Rubin A., Batushansky I., Asadi A., O'Dwyer S., Quiskamp N., Mojibian M., Albrecht T. et al. (2014). Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 132, 1121-1133. 10.1038/nbt.3033 [DOI] [PubMed] [Google Scholar]
- Roccisana J., Reddy V., Vasavada R. C., Gonzalez-Pertusa J. A., Magnuson M. A. and Garcia-Ocaña A. (2005). Targeted inactivation of hepatocyte growth factor receptor c-met in beta-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of beta-cell mass. Diabetes 54, 2090-2102. 10.2337/diabetes.54.7.2090 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Diaz R., Dando R., Jacques-Silva M. C., Fachado A., Molina J., Abdulreda M. H., Ricordi C., Roper S. D., Berggren P.-O. and Caicedo A. (2011). Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 17, 888-892. 10.1038/nm.2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis J. M. and Habener J. F. (2007). Snail2, a mediator of epithelial-mesenchymal transitions, expressed in progenitor cells of the developing endocrine pancreas. Gene Expr. Patterns GEP 7, 471-479. 10.1016/j.modgep.2006.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson E. J., Kim S. K. and Nusse R. (2002). Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118-1120. 10.1126/science.1070058 [DOI] [PubMed] [Google Scholar]
- Sanvito F., Herrera P. L., Huarte J., Nichols A., Montesano R., Orci L. and Vassalli J. D. (1994). TGF-beta 1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development 120, 3451-3462. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S. and Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671-675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serup P. (2012). Signaling pathways regulating murine pancreatic development. Semin. Cell Dev. Biol. 23, 663-672. 10.1016/j.semcdb.2012.06.004 [DOI] [PubMed] [Google Scholar]
- Shelly M., Cancedda L., Lim B. K., Popescu A. T., Cheng P., Gao H. and Poo M. (2011). Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron 71, 433-446. 10.1016/j.neuron.2011.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih H. P., Kopp J. L., Sandhu M., Dubois C. L., Seymour P. A., Grapin-Botton A. and Sander M. (2012). A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development 139, 2488-2499. 10.1242/dev.078634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubin N., Tabin C. and Carroll S. (1997). Fossils, genes and the evolution of animal limbs. Nature 388, 639-648. 10.1038/41710 [DOI] [PubMed] [Google Scholar]
- Shubin N., Tabin C. and Carroll S. (2009). Deep homology and the origins of evolutionary novelty. Nature 457, 818-823. 10.1038/nature07891 [DOI] [PubMed] [Google Scholar]
- Steiner D. J., Kim A., Miller K. and Hara M. (2010). Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets 2, 135-145. 10.4161/isl.2.3.11815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S. C. and Tsai L.-H. (2011). Cyclin-dependent kinases in brain development and disease. Annu. Rev. Cell Dev. Biol. 27, 465-491. 10.1146/annurev-cellbio-092910-154023 [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Rodriguez R. T., McLean G. W. and Kim S. K. (2007). Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc. Natl. Acad. Sci. USA 104, 175-180. 10.1073/pnas.0609490104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Benitez C. M., Ghodasara A., Liu L., McLean G. W., Lee J., Blauwkamp T. A., Nusse R., Wright C. V. E., Gu G. et al. (2013). Reconstituting pancreas development from purified progenitor cells reveals genes essential for islet differentiation. Proc. Natl. Acad. Sci. USA 110, 12691-12696. 10.1073/pnas.1304507110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Fournier A., Nakamura F., Wang L.-H., Murakami Y., Kalb R. G., Fujisawa H. and Strittmatter S. M. (1999). Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99, 59-69. 10.1016/S0092-8674(00)80062-8 [DOI] [PubMed] [Google Scholar]
- Takashima S., Kitakaze M., Asakura M., Asanuma H., Sanada S., Tashiro F., Niwa H., Miyazaki J.-I., Hirota S., Kitamura Y. et al. (2002). Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc. Natl. Acad. Sci. USA 99, 3657-3662. 10.1073/pnas.022017899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L., Artigiani S., Chen H., He Z., Ming G.-I., Song H., Chedotal A., Winberg M. L., Goodman C. S., Poo M. et al. (1999). Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99, 71-80. 10.1016/S0092-8674(00)80063-X [DOI] [PubMed] [Google Scholar]
- Tomer R., Ye L., Hsueh B. and Deisseroth K. (2014). Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat. Protoc. 9, 1682-1697. 10.1038/nprot.2014.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T. S., Kolodkin A. L. and Bharadwaj R. (2007). Semaphorin regulation of cellular morphology. Annu. Rev. Cell Dev. Biol. 23, 263-292. 10.1146/annurev.cellbio.22.010605.093554 [DOI] [PubMed] [Google Scholar]
- Tran T. S., Rubio M. E., Clem R. L., Johnson D., Case L., Tessier-Lavigne M., Huganir R. L., Ginty D. D. and Kolodkin A. L. (2009). Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature 462, 1065-1069. 10.1038/nature08628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulachan S. S., Tei E., Hembree M., Crisera C., Prasadan K., Koizumi M., Shah S., Guo P., Bottinger E. and Gittes G. K. (2007). TGF-beta isoform signaling regulates secondary transition and mesenchymal-induced endocrine development in the embryonic mouse pancreas. Dev. Biol. 305, 508-521. 10.1016/j.ydbio.2007.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meulen T., Donaldson C. J., Cáceres E., Hunter A. E., Cowing-Zitron C., Pound L. D., Adams M. W., Zembrzycki A., Grove K. L. and Huising M. O. (2015). Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat. Med. 21, 769-776. 10.1038/nm.3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noorden S. and Falkmer S. (1980). Gut-islet endocrinology-some evolutionary aspects. Invest. Cell Pathol. 3, 21-35. [PubMed] [Google Scholar]
- Walz A., Rodriguez I. and Mombaerts P. (2002). Aberrant sensory innervation of the olfactory bulb in neuropilin-2 mutant mice. J. Neurosci. Off. J. Soc. Neurosci. 22, 4025-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells N. K. and Cohen J. H. (1967). Early pancreas organogenesis: morphogenesis, tissue interactions, and mass effects. Dev. Biol. 15, 237-270. 10.1016/0012-1606(67)90042-5 [DOI] [PubMed] [Google Scholar]
- Winberg M. L., Noordermeer J. N., Tamagnone L., Comoglio P. M., Spriggs M. K., Tessier-Lavigne M. and Goodman C. S. (1998). Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell 95, 903-916. 10.1016/S0092-8674(00)81715-8 [DOI] [PubMed] [Google Scholar]
- Yang Y. H. C., Szabat M., Bragagnini C., Kott K., Helgason C. D., Hoffman B. G. and Johnson J. D. (2011). Paracrine signalling loops in adult human and mouse pancreatic islets: netrins modulate beta cell apoptosis signalling via dependence receptors. Diabetologia 54, 828-842. 10.1007/s00125-010-2012-5 [DOI] [PubMed] [Google Scholar]
- Yebra M., Montgomery A. M. P., Diaferia G. R., Kaido T., Silletti S., Perez B., Just M. L., Hildbrand S., Hurford R., Florkiewicz E. et al. (2003). Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev. Cell 5, 695-707. 10.1016/S1534-5807(03)00330-7 [DOI] [PubMed] [Google Scholar]
- Yoshitomi H. and Zaret K. S. (2004). Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development 131, 807-817. 10.1242/dev.00960 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.