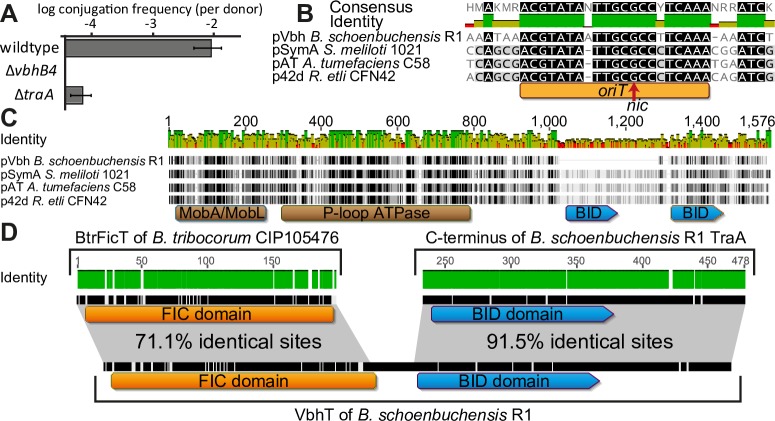
Fig 4. pVbh as a conjugative replicon with composite toxin VbhT.
(A) Filter matings of B. schoenbuchensis R1 donors and B. henselae recipients revealed that pVbh conjugates at a frequency of ca. 1% per donor. Conjugative transfer depended on a functional Vbh T4SS (vbhB4 mutant) and its Dtr functions (traA mutant). Data points and error bars represent mean and standard deviation of at least three independent experiments. (B) The origin of conjugative DNA transfer (oriT, orange bar) on pVbh was inferred by comparison to closely related rhizobial plasmids where this sequence and the actual site of relaxase cleavage (nic) had been experimentally determined [16]. All these plasmids invariably encode oriT between the traA relaxase and the traCD relaxasome components in the dtr region (see Fig 2). (C) Domain composition and sequence alignment of the TraA relaxase of pVbh with relaxases of closely related rhizobial conjugation systems. (D) The alignment of BtrFicT, VbhT, and TraA protein sequences shows that VbhT is a composite protein with an N-terminal FIC domain closely related to FicT toxins of Bartonella and a C-terminal secretion signal virtually identical to homologous sequence of the TraA relaxase.