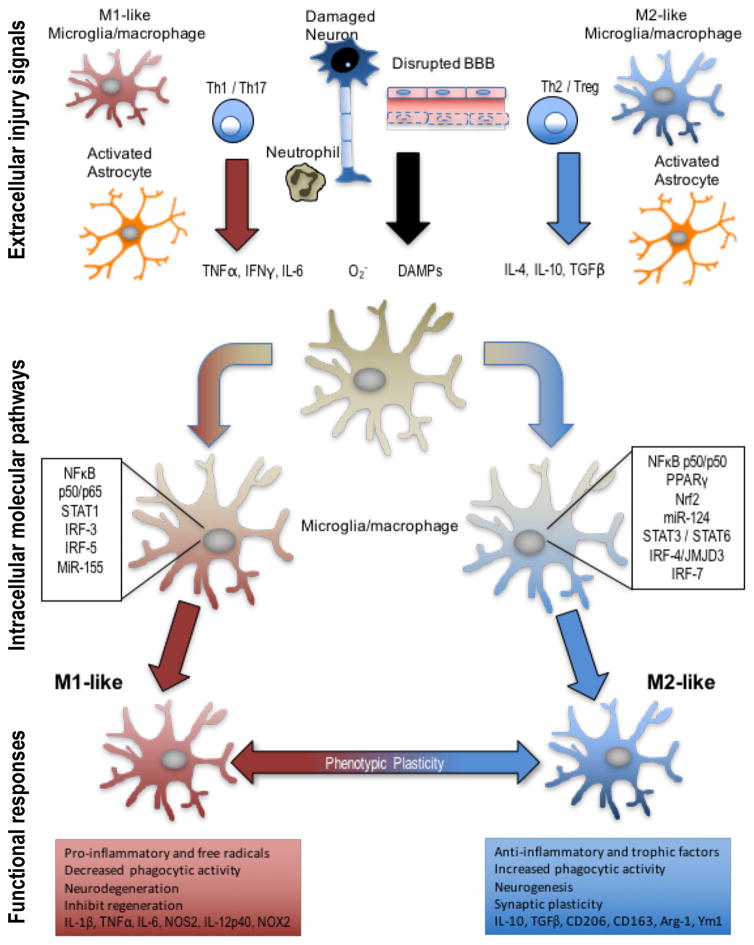
Figure 2. Extracellular injury signals and intracellular molecular pathways control polarization of microglia and macrophages following TBI.
Molecular signals from injured tissue can drive phenotypic and functional responses in microglia/macrophages after TBI. DAMPs released by injured neurons, pro-inflammatory or oxidative mediators released by infiltrating immune cells including TNFα, IFNγ, IL-6, and O2− can polarize cells towards an M1-like phenotype. M1-like populations are characterized by expression of proteins such as IL-1β, TNFα, IL-6, NOS2, IL-12p40, and NOX2. Molecular pathways that regulate the M1-phenotype include STAT1, IRF-3/5, NFκB p50/p65 and miR-155, among others. M1-like microglia and macrophages release pro-inflammatory factors and free radicals that promote chronic neuroinflammation, oxidative stress and neurodegeneration, and inhibit regeneration. In response to anti-inflammatory and neurotrophic signals microglia and macrophages can be polarized towards an M2-like phenotype, characterized by expression of proteins such as CD206, CD163, Arginase 1, FCγR, Ym1, IL-10, and TGFβ. Molecular pathways that regulate M2-like phenotypic transitions include STAT6/3, IRF-4/7, NF-κB p50/p50, Nrf2 and miR-124, among others. M2-like microglia and macrophages release anti-inflammatory and trophic factors that resolve inflammation. They also have increased phagocytic activity, and improve brain repair by modulating neurogenesis, axonal regeneration, synaptic plasticity, and angiogenesis. Microglia and macrophages demonstrate significant plasticity and can switch between M1- and M2-like phenotypes. Moreover, it is recognized that following TBI they present mixed phenotypes during the acute phase post-injury, and transitions to an M1-like dominant phenotype in the chronic phase after TBI.
Abbreviations: TBI, traumatic brain injury; DAMP, damage-associated molecular pattern; TNF, tumor necrosis factor; IFN, interferon; IL, interleukin; NOS, nitric oxide synthase; NOX, nicotinamide adenine dinucleotide phosphate oxidase; STAT, signal transducer and activator of transcription; IRF, interferon regulatory factor; CD, cluster of differentiation; TGF, transforming growth factor; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells