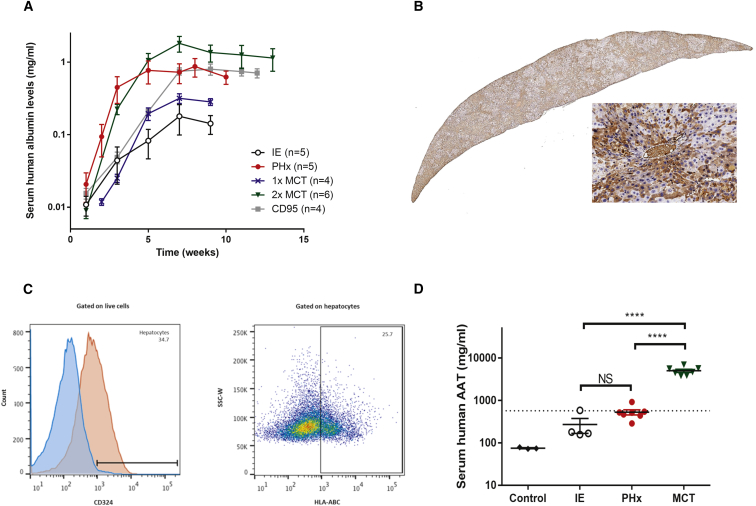
Figure 4.
Optimization of the Human Liver Xenograft Model
(A) Repopulation rate was assessed after various challenges. The challenges compared were as follows: intrasplenic engraftment alone (IE, white circles), intrasplenic injection with partial hepatectomy (PHx, red circles), a single intraperitoneal dose of 50 mg/kg monocrotaline (MCT) at 7 days pre-engraftment (1× MCT, blue crosses), a double intraperitoneal dose of 50 mg/kg MCT at 14 and 7 days pre-engraftment (2× MCT, green triangles), and a dose of 1 μg CD95 intravenously (i.v.) at the time of engraftment (CD95, gray squares). Serum was collected biweekly post-engraftment, and human albumin levels were quantified as a measure of repopulation. Data are plotted as mean ± SEM. **Statistically significant differences between groups. (B) Liver tissue was harvested at 10 weeks post-engraftment from MCT-treated mice and stained for human albumin; one representative animal is shown. (C) Hepatocytes of human origin were quantified by flow cytometry. Hepatocytes were gated as CD324+ cells, and human hepatocytes are defined as cells expressing the human HLA-ABC marker within the CD324+ subset. One representative animal is shown. Percentages are indicated in the histogram and dot plot. (D) Serum human AAT levels were quantified by ELISA in various treatment groups at 10 weeks post-engraftment. Data are plotted as mean ± SEM. The dotted line denotes the therapeutic threshold at 572 μg/mL. ****Statistically significant difference between groups (two-way unpaired t test).