Main Text
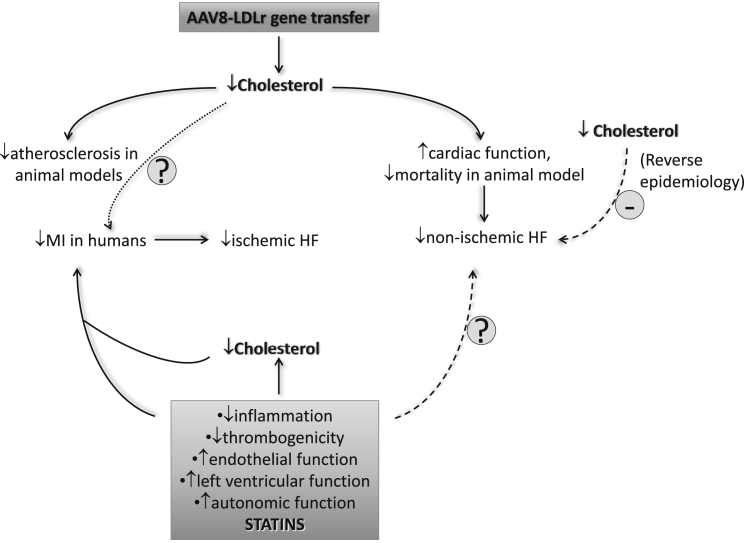
The relationship between plasma cholesterol levels and non-ischemic heart failure has been controversial. In this issue of Molecular Therapy, Muthuramu et al. 1 address this issue by modulating plasma lipid levels by gene transfer of low-density lipoprotein receptor using an AAV8 vector (AAV8-LDLr) in a mouse model of cardiac pressure overload induced by transverse aortic constriction (TAC). AAV8-LDLr gene transfer induced a strong reduction of plasma cholesterol and lipoprotein levels, which then resulted in the attenuation of cardiac hypertrophy and decreased lung congestion as well as mortality after TAC1 (Figure 1). The reduced plasma cholesterol achieved through this approach improved cardiac function and reduced interstitial and perivascular fibrosis. At the molecular level, AAV8-LDLr gene transfer dampened oxidative stress and myocardial apoptosis, perhaps through the improvement of cellular metabolic adaptations induced in the heart by pressure overload.
Figure 1.
Cholesterol Lowering and Heart Failure
Low plasma cholesterol levels are associated with an improved outcome in ischemic heart failure (HF) but appear to be linked to a worse prognosis in patients with non-ischemic HF. In humans, statin therapy reduces the risk of new onset HF in patients with CAD through several mechanisms, while the effect on non-ischemic HF is still unclear. On the contrary, AAV8-LDLr gene transfer lowers plasma cholesterol levels in a mouse model of non-ischemic heart failure and reduces HF-dependent mortality in mice through the improvement of cellular metabolic adaptations induced in the heart by pressure overload. AAV8-LDLr gene therapy is under evaluation in patients with familial hypercholesterolemia and might contribute to further understand the role of this approach on heart failure.
Ischemic heart disease represents the most common cause of heart failure, but non-ischemic cardiomyopathies may result in heart failure as well. There is conflicting evidence for a relationship between plasma cholesterol levels and heart failure, as several studies have reported different patterns of plasma cholesterol in patients with ischemic and non-ischemic disease. Low levels of total cholesterol appear to be associated with an improved outcome in ischemic heart failure, but in patients with non-ischemic disease, they appear to predict a worse prognosis (referred to as “reverse epidemiology”)2, 3, 4 (Figure 1). In the Framingham study, plasma total cholesterol was not significantly related to the occurrence of congestive heart failure, whereas an increased total cholesterol to high-density lipoprotein (HDL) ratio predicted disease incidence.5
Despite the inconsistent data on the relationship between cholesterol and heart failure incidence, statin-induced cholesterol lowering has been shown to reduce the risk of new-onset heart failure in patients with coronary artery disease (CAD).5 Initially, two large trials failed to show an increased survival in chronic heart failure patients treated with statins.5 A retrospective analysis of the CORONA (Controlled Rosuvastatin Multinational Study in Heart Failure) study later demonstrated that rosuvastatin treatment significantly decreased the risk of hospitalization.6 Similar findings were observed following post hoc analysis of the 4S study with simvastatin7 and the PROVE IT-TIMI 22 study with atorvastatin.8 Large meta-analyses further demonstrated that statins persistently decreased the incidence of rehospitalization for heart failure in chronic patients9 and patients with non-ischemic disease.10 Importantly, this beneficial effect was confirmed in national registry studies in patients with or without preserved ejection fraction (EF),11, 12 and statins were shown to attenuate the strength of the inverse association between total cholesterol levels and survival.4
Based on these observations, the beneficial effects of statins have been attributed to their anti-inflammatory properties, counter-regulatory action on the renin-angiotensin-aldosterone and sympathetic systems, or positive effects on cardiac hypertrophy and fibrosis rather than to their lipid-lowering activity13 (Figure 1). Beyond the mechanism, it is reasonable to speculate that statins exert their protective effect before heart failure develops by reducing the risk of ischemic heart disease, whereas they may be neutral when disease is already manifest. The study by Muthuramu et al., however, demonstrates in animal models that lipid lowering, other than that achieved with statins, is beneficial on non-ischemic heart failure development. These observations nevertheless leave some key questions unsolved. Could cholesterol reduction be the common driver of this effect? Statins lack a robust lipid lowering activity in animal models, suggesting that, at least in animal models, their protective effect on heart failure14, 15 might occur through mechanisms other than those observed following AVV8 gene therapy.
Do increased plasma lipoprotein levels foster cardiac cell proliferation and in turn hypertrophy? Muthuramu et al.1 do not show whether LDLR knockout (KO) mice with TAC develop cardiac hypertrophy to an extent greater than C57BL6 TAC mice or whether LDLR KO mice fed a normal or lipid-enriched diet present a similar degree of hypertrophy. It must be acknowledged, however, that the mouse presents some diversity in plasma lipid and lipoprotein distribution, with HDL being the main class of lipoprotein that transports cholesterol compared to humans, where low density lipoproteins dominate. Muthuramu et al.1 used mice lacking the LDLR which, when fed a hyperlipidic diet, present a lipid and lipoprotein profile that is closer to that of humans. Nevertheless, testing AAV8-LDLr gene transfer in more humanized animal models for hypercholesterolemia, such as the LDLR-KO Apob100/100 Tg mouse or the ApoE*3Leiden/CETP Tg mouse, might have further supported a role for plasma cholesterol reduction in non-ischemic heart failure with a clinical perspective.
Could the beneficial effect be peculiar to the animal model used? TAC is a procedure commonly used in the mouse for pressure overload-induced cardiac hypertrophy and heart failure. While heart hypertrophy initially develops to compensate the overload, the chronic hemodynamic changes become maladaptive, resulting in cardiac dilatation and heart failure. This is a robust model that mimics the human pathology and provides a more reproducible model of heart failure development compared to complete occlusion of the left anterior descending (LAD) coronary artery, thus enforcing the findings of the present work.
Is the AAV8-LDLr gene transfer ready for translation in patients with heart failure? Not yet. In fact, whereas AAV8-LDLr gene therapy is already under investigation in patients with familial hypercholesterolemia, with the aim of restoring LDLR activity (NCT02651675) and thus limiting atherosclerosis and the risk of premature death for myocardial infarction, expansion of the indication of AAV8-LDLr gene therapy to heart failure is far from ready for clinical testing. If induction of the LDLR in the liver and the consequent lowering of plasma cholesterol are key, as it appears in this study, then statins should work as well.
Despite these aspects, the work by Muthuramu et al.1 paves the way for addressing the role of lipid-lowering approaches that increase LDLR expression/function in improving non-ischemic heart failure. In an era where many pharmacological approaches for the treatment of dyslipidemia are emerging, further analyses of the data from the trials with ezetimibe, PCSK9, or CETP inhibitors on non-ischemic heart failure incidence/progression are warranted to support a beneficial effect in patients that might be related to the lipid lowering activity.
References
- 1.Muthuramu I., Amin R., Postnov A., Mishra M., Aboumsallem J.P., Dresselaers T., Himmelreich U., Van Veldhoven P.P., Gheysens O., Jacobs F., De Geest B. Cholesterol-lowering gene therapy counteracts the development of non-ischemic cardiomyopathy in mice. Mol. Ther. 2017;25:2513–2525. doi: 10.1016/j.ymthe.2017.07.017. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afsarmanesh N., Horwich T.B., Fonarow G.C. Total cholesterol levels and mortality risk in nonischemic systolic heart failure. Am. Heart J. 2006;152:1077–1083. doi: 10.1016/j.ahj.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Sakatani T., Shirayama T., Suzaki Y., Yamamoto T., Mani H., Kawasaki T., Sugihara H., Matsubara H. The association between cholesterol and mortality in heart failure. Comparison between patients with and without coronary artery disease. Int. Heart J. 2005;46:619–629. doi: 10.1536/ihj.46.619. [DOI] [PubMed] [Google Scholar]
- 4.Fröhlich H., Raman N., Täger T., Schellberg D., Goode K.M., Kazmi S., Grundtvig M., Hole T., Cleland J.G.F., Katus H.A. Statins attenuate but do not eliminate the reverse epidemiology of total serum cholesterol in patients with non-ischemic chronic heart failure. Int. J. Cardiol. 2017;238:97–104. doi: 10.1016/j.ijcard.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 5.Horwich T. Low-density lipoprotein in the setting of congestive heart failure: is lower really better? Curr. Atheroscler. Rep. 2009;11:343–349. doi: 10.1007/s11883-009-0052-4. [DOI] [PubMed] [Google Scholar]
- 6.Rogers J.K., Jhund P.S., Perez A.C., Böhm M., Cleland J.G., Gullestad L., Kjekshus J., van Veldhuisen D.J., Wikstrand J., Wedel H. Effect of rosuvastatin on repeat heart failure hospitalizations: the CORONA Trial (Controlled Rosuvastatin Multinational Trial in Heart Failure) JACC Heart Fail. 2014;2:289–297. doi: 10.1016/j.jchf.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen T.R. Coronary artery disease: the Scandinavian Simvastatin Survival Study experience. Am. J. Cardiol. 1998;82(10B):53T–56T. doi: 10.1016/s0002-9149(98)00727-9. [DOI] [PubMed] [Google Scholar]
- 8.Scirica B.M., Morrow D.A., Cannon C.P., Ray K.K., Sabatine M.S., Jarolim P., Shui A., McCabe C.H., Braunwald E., PROVE IT-TIMI 22 Investigators Intensive statin therapy and the risk of hospitalization for heart failure after an acute coronary syndrome in the PROVE IT-TIMI 22 study. J. Am. Coll. Cardiol. 2006;47:2326–2331. doi: 10.1016/j.jacc.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 9.Wang J.Q., Wu G.R., Wang Z., Dai X.P., Li X.R. Long-term clinical outcomes of statin use for chronic heart failure: a meta-analysis of 15 prospective studies. Heart Lung Circ. 2014;23:105–113. doi: 10.1016/j.hlc.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Deo S.V., Rababa’h A., Altarabsheh S.E., Lim J.Y., Cho Y.H., Park S.J. Statin therapy improves long-term survival in non-ischaemic cardiomyopathy: a pooled analysis of 4500 patients. Heart Lung Circ. 2014;23:985–987. doi: 10.1016/j.hlc.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Alehagen U., Benson L., Edner M., Dahlström U., Lund L.H. Association Between Use of Statins and Mortality in Patients With Heart Failure and Ejection Fraction of ≥50. Circ Heart Fail. 2015;8:862–870. doi: 10.1161/CIRCHEARTFAILURE.115.002143. [DOI] [PubMed] [Google Scholar]
- 12.Alehagen U., Benson L., Edner M., Dahlström U., Lund L.H. Association between use of statins and outcomes in heart failure with reduced ejection fraction: prospective propensity score matched cohort study of 21 864 patients in the Swedish Heart Failure Registry. Circ Heart Fail. 2015;8:252–260. doi: 10.1161/CIRCHEARTFAILURE.114.001730. [DOI] [PubMed] [Google Scholar]
- 13.Ramasubbu K., Estep J., White D.L., Deswal A., Mann D.L. Experimental and clinical basis for the use of statins in patients with ischemic and nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 2008;51:415–426. doi: 10.1016/j.jacc.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Saka M., Obata K., Ichihara S., Cheng X.W., Kimata H., Nishizawa T., Noda A., Izawa H., Nagata K., Murohara T., Yokota M. Pitavastatin improves cardiac function and survival in association with suppression of the myocardial endothelin system in a rat model of hypertensive heart failure. J. Cardiovasc. Pharmacol. 2006;47:770–779. doi: 10.1097/01.fjc.0000211791.22411.0d. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Garre D., González-Rubio M.L., Muñoz-Pacheco P., Caro-Vadillo A., Aragoncillo P., Fernández-Cruz A. Rosuvastatin added to standard heart failure therapy improves cardiac remodelling in heart failure rats with preserved ejection fraction. Eur. J. Heart Fail. 2010;12:903–912. doi: 10.1093/eurjhf/hfq101. [DOI] [PubMed] [Google Scholar]