Abstract
The prognostic role of COX-2 expression in ovarian cancer patients has been studied for years, while results remain controversial. Thus we performed a meta-analysis to evaluate the prognostic impact of COX-2 expression on survival of ovarian cancer patients. The databases PubMed, Embase and CNKI were searched. Summary hazard ratio (HR) and 95% confidence intervals (CIs) were calculated to analyze the correlations between COX-2 expression and overall survival (OS), and disease-free survival (DFS). A total of 1,867 patients from 18 studies were enrolled in the final analysis. The results showed that patients with higher COX-2 expression had a poor OS (HR: 1.48; 95% CI: 1.19-1.85) and DFS (HR: 1.81, 95% CI: 1.28-2.55). Subgroup analysis showed that there had significant associations between COX-2 expression and survival rate in most of the subgroups. Furthermore, there were significant associations between COX-2 expression and several clinical parameters such as FIGO stage, histological type and age. These results showed the patients with higher COX-2 expression had a significantly poorer survival rate, COX-2 expression had the potential to be a prognostic marker of ovarian cancer.
Keywords: COX-2, ovarian cancer, meta-analysis, prognosis
INTRODUCTION
Ovarian cancer ranks the fifth as a cause of neoplastic death among women, there would be more than 22,000 new cases and over 14,000 deaths in the United States in 2017 [1]. Because early-stage tumors are typically asymptomatic, most patients have advanced stage at the time of diagnosis, resulting in a poorer long-time survival [2, 3]. The overall 5-year survival rate of ovarian cancer is just approximately 30% [4]. Given the poor survival rates of ovarian cancer, it is necessary to identify prognostic biomarkers for effectively evaluating the outcomes of the patients.
The cyclooxygenase-2 (COX-2) is called the prostaglandin-endoperoxide synthase-2 (PTGS-2) too, it involved in the processes of inflammatory and oncogenic [5, 6]. Furthermore, COX-2 dependent prostaglandin release can inhibit antigen presentation and immune activation during carcinogenesis [7]. COX-2 overexpression was found in most solid tumors, such as breast, colorectal, lung, pancreatic, liver, as well as ovarian cancer [8–11]. And some studies have reported the prognostic role of the expression of COX-2 in ovarian cancer, while the results are varying and sometimes conflicting. In order to clarify the issues, we collected the eligible articles and performed a meta-analysis to assess the prognostic impact of COX-2 expression in the patients with ovarian cancer.
RESULTS
Literature searching and study characteristics
By the keywords searching, a total of 547 publications were found. Based upon the selection criteria, a total of 18 publications were identified and included in the final analysis [12–29]. The details of the procedures of literature screening were shown in Figure 1. The total number of ovarian cancer patients included in the meta-analysis was 1,867, the mean sample size was 103 (ranges from 32 to 442). Among them, 18 studies reported the overall survival (OS) and 4 for disease-free survival (DFS). In the study of Ferrandina [28], they presented the data in two subgroups, so we treated the study as two datasets. We extracted hazard ratio (HR) and a 95% confidence interval (CI) from the Kaplan-Meier curves in 10 articles. The main characteristics of the included studies were listed in Table 1.
Figure 1. Flow chart of the literature search.
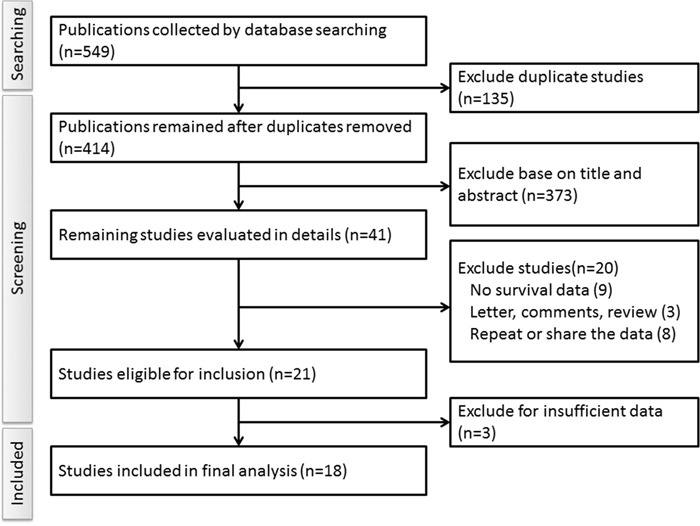
Table 1. Main characteristics of the studies included in this meta-analysis.
| Study ID | Country | Sample size | Median or mean age/range(year) | FIGO stage | Histological subtype | Follow-up time (months) | COX-2 detection method | High expression cut-off level | Number of high expression patients | Outcome (OS/DFS) | Study quality | Source of HR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ali-Fehmi (2011) | USA | 126 | 57.6 | I-IV | serous | 54 (1-235) | IHC | Staining intensity ≥2 and stained cells >10% or staining intensity ≥1 and stained cells >50% staining intensity ≥1 and stained cells >50% | 96 | OS | 9 | R |
| Denkert (2002) | Germany | 86 | NA | I-IV | Serous, undifferentiated, nonserous | 32.5 (0.3-121.7) | IHC | Diffuse staining or a focal expression in several clusters of cells | 36 | OS | 5 | R |
| Seo (2004) | South Korea | 64 | 51 | I-IV | serous, metrioid, mucinous | 56 (6-68) | IHC | >5% of cells were positively stained for mucinous or >30% for serous and endometrioid | 64 | OS | 8 | R |
| Raspollini (2004) | Italy | 78 | 58 | III | serous | 47 (3-204) | IHC | Positive staining >10% of the total tumor area of intensity of staining scored ≥2 | 54 | OS/DFS | 8 | R |
| Ferrero (2011) | Italy | 113 | 62 | II-IV | Serous, mucinous, endometrioid, undifferentiated | NA | IHC | Staining intensity ≥2 and >10% stained cells or staining intensity ≥1 and >50% stained cells | 45 | OS | 7 | R |
| Surowiak (2006) | Poland | 43 | NA | III | Serous, endometrioid | NA | IHC | Stained in all tumors cells or in numerous cell clumps | 19 | OS/DFS | 6 | E |
| Wang (2011) | China | 147 | 43.15 | I-IV | Serous, mucinous and others | NA | IHC | Staining intensity ≥2 or percentage of stained cells ≥ 30% | 109 | OS | 5 | R |
| Athanassiadou (2008) | Greece | 100 | 62 | I-IV | Serous, mucinous, endometrioid, undifferentiated | 67.12 | IHC | Staining reaction >10% | 56 | OS | 6 | E |
| Erkinheimo (2004) | Finland | 442 | 57 | I-IV | Serous | 5.2 years (0.4-36.1) | IHC | Staining in >10% cancer cells | 310 | OS | 9 | E |
| Khalifeh (2004) | USA | 96 | 62 | III, IV | Serous | 35.3 | IHC | Intensity 2 or 3 and >10% and /or intensity 1,2 or 3 and >50% | 65 | OS | 7 | E |
| Steffensen (2007) | Denmark | 160 | 54.5 | II-IV | Serous, mucinous, endometrioid, undifferentiated and others | more than 10 years | IHC | >10% of the total tumor area showing moderate or strong immunostaining | 32 | OS | 7 | R |
| Magnowska (2014) | Poland | 65 | NA | NA | Serous and others | 37.2 (24-74) | IHC | Immunoreactivity Score >6 | 33 | OS/DFS | 5 | R |
| Taskin (2012) | Turkey | 32 | 58.63 | II-III | Serous | 33.7 (8-124) | IHC | The multiplied staining intensity and stained cell percent >3 | 15 | OS | 7 | E |
| Lou (2004) | China | 70 | 54 | I-IV | Serous, mucinous, endometrioid, undifferentiated and others | 31 (5-71) | IHC | Staining in >10% cancer cells | 42 | OS | 6 | E |
| Lee (2006) | USA | 54 | 51 | I-IV | Serous, mucinous, endometrioid and clear cell | 67 (3-119) | IHC | Staining intensity of 2 or 3 and >10% stained cells or an intensity 1 and >50% stained cells | 42 | OS | 7 | E |
| Fujimoto (2006) | Japan | 60 | NA | I-III | Serous, mucinous, endometrioid | up to 24 months | ELISA | >14 ng/mg protein | 30 | OS | 6 | E |
| Ferrandina (2002) | Italy | 87 | 57 | III, IV | Serous, mucinous, endometrioid, undifferentiated and others | 25 (4-147) | IHC | >10% of the total tumor area or intensity of staining ≥2 | 39 | OS/DFS | 6 | E |
| Ozuysal (2009) | Turkey | 44 | 54.2 | I-IV | Serous | 40 | IHC | Staining intensity ≥ 2 and percentage >10% or staining intensity ≥ 1 and percentage >50% | 17 | OS | 7 | E |
IHC: immunohistochemistry; NA: not available; OS: overall survival; DFS: disease-free survival; EILSA: enzyme-linked immunosorbent assay; R: reported in the articles; E: estimated from Kaplan-Meier plots.
COX-2 expression and OS
Firstly, we analyzed the impact of COX-2 expression levels on OS in 18 studies. The random-effects model was used to estimate the pooled HRs and the respective 95% CI. The results showed that the patients with higher COX-2 expression had a significantly increased risk of death than the patients with lower COX-2 expression (Figure 2, HR: 1.48; 95% CI: 1.19-1.85). Then, we performed subgroup analysis according to study quality, sample size, follow-up time, histology subtypes, regions of the study and calculation methods of HR. Based on the region of study, we found patients with higher COX-2 expression had a poor prognosis in European and Asian studies. When subgroups were stratified by the statistical analysis methodology, the results demonstrated that the survival rates had an obvious distinction between lower and higher expression groups both by univariable analysis and multivariable analysis. When restricted to the histology types of the cancer, we found that patients with higher COX-2 expression had a poor survival rate not only in serous ovarian but also in the total types of ovarian cancer. No matter the length of the follow-up time, the patients with a higher COX-2 expression had a lower overall survival rates. But the results did not have statistical significant in two subgroups. All of the details were listed in Table 2.
Figure 2. Forest plots of hazard ratios (HRs) for the association between COX-2 expression and overall survival (OS) in ovarian cancer patients.
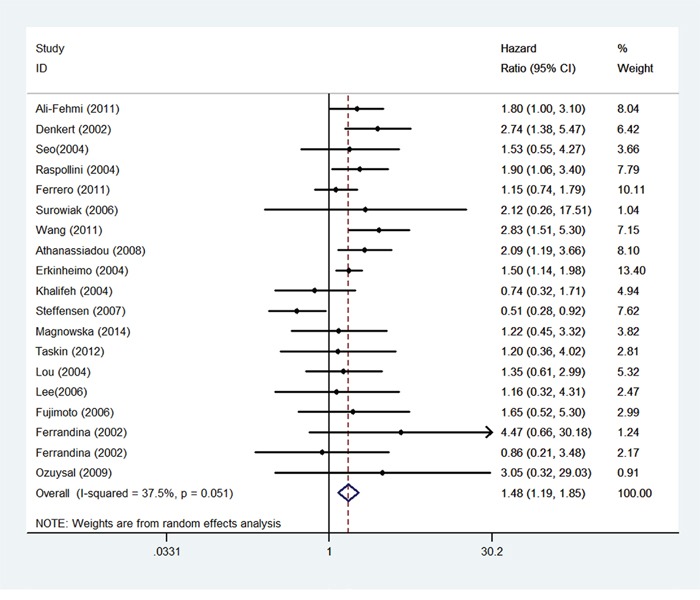
The 95% confidence intervals (CI) for individual studies are represented by a horizontal line and by a diamond for pooled effect. CI denotes confidence interval.
Table 2. Main results of the meta-analysis.
| Categories | Number of datasets | HR | 95%CI | P | Degree of heterogeneity (I2 statistics; %) |
|---|---|---|---|---|---|
| OS | 19 | 1.48 | 1.19-1.85 | <0.001 | 37.5 |
| Study quality | |||||
| Score ≥ 7 | 10 | 1.24 | 0.93-1.65 | 0.136 | 44.3 |
| < 7 | 9 | 2.04 | 1.53-2.71 | <0.001 | 0 |
| Sample size | |||||
| ≥ 100 | 6 | 1.44 | 0.98-2.13 | 0.065 | 74.3 |
| < 100 | 13 | 1.57 | 1.19-2.07 | 0.001 | 0 |
| Duration of follow-up (Months) | |||||
| > 36 | 9 | 1.42 | 1.04-1.95 | 0.029 | 48.7 |
| ≤ 36 | 10 | 1.56 | 1.11-2.20 | 0.01 | 31.2 |
| Histology types | |||||
| All | 13 | 1.49 | 1.06-2.09 | 0.021 | 50.9 |
| Serous | 6 | 1.52 | 1.22-1.88 | <0.001 | 0 |
| Region | |||||
| European | 12 | 1.44 | 1.07-1.95 | 0.016 | 48.3 |
| North American | 3 | 1.25 | 0.69-2.26 | 0.456 | 33.8 |
| Asian | 4 | 1.95 | 1.29-2.95 | 0.002 | 0 |
| Analysis type | |||||
| Multivariate | 8 | 1.52 | 1.01-2.28 | 0.044 | 68.2 |
| Univariate | 11 | 1.49 | 1.21-1.84 | <0.001 | 0 |
HR: hazard ratio; CI: confidence interval; OS: overall survival.
COX-2 expression and DFS
In the enrolled studies, there were four studies reported the associations between COX-2 expression and DFS [15, 17, 23, 28], in which, the study of Ferrandina included two datasets. The results indicated that the patients with higher COX-2 expression had a poor disease-free survival (Figure 3, HR: 1.81, 95% CI: 1.28-2.55).
Figure 3. Forest plots of hazard ratios (HRs) for the association between COX-2 expression and disease-free survival (DFS) in ovarian cancer patients.
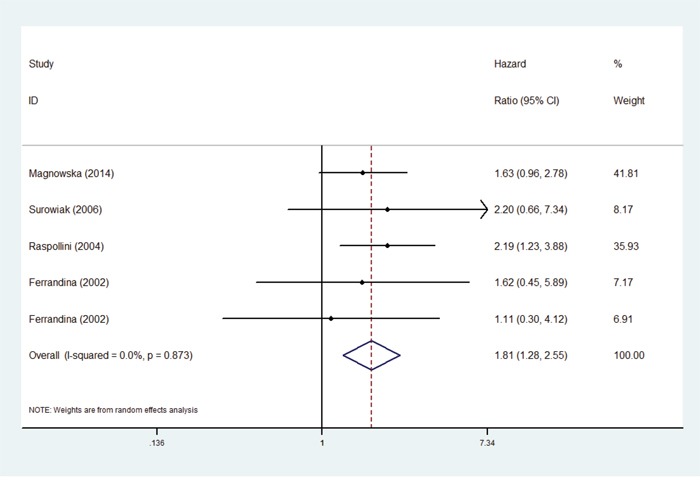
The 95% confidence intervals (CI) for individual studies are represented by a horizontal line and by a diamond for pooled effect. CI denotes confidence interval.
Sensitivity analysis and publication bias
In order to evaluate the influence of the single study to the final results, sensitivity analysis was carried out by deleted single study each time. As shown in Figure 4, there was no significant change on the results by removing any of the studies, which mean that our results were reliable. Begg's funnel plots were constructed to evaluate the publication bias; we did not find the sign of publication bias by the shapes of the plots (Figure 5). The results of Egger's test showed there were no publication bias, too (P = 0.892 for OS; P = 0.599 for DFS).
Figure 4.
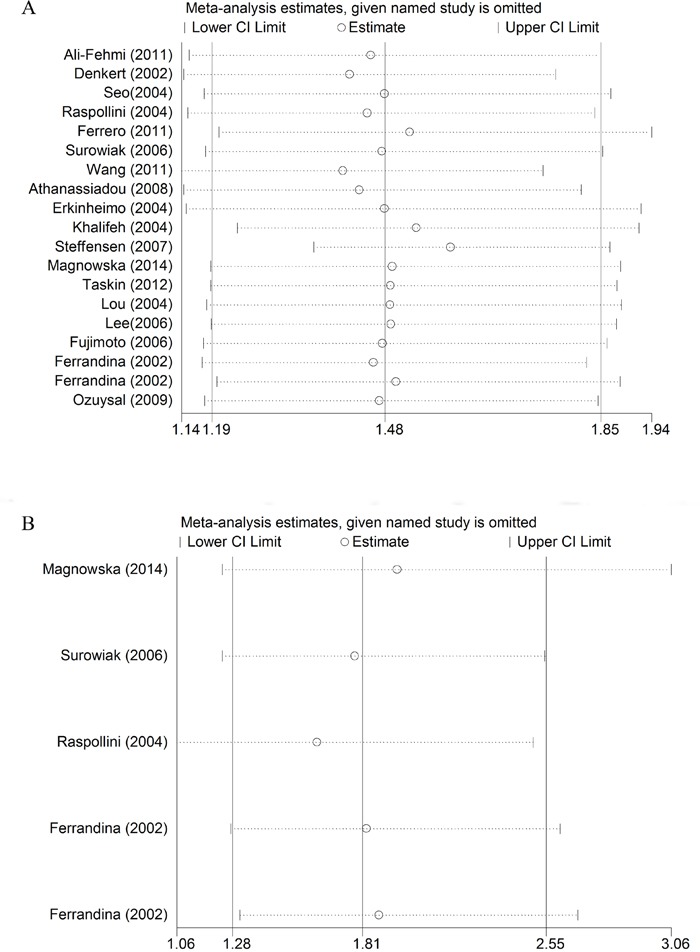
Sensitive analysis of the pooled hazard ratio for OS (A) and DFS (B). Meta-analysis random effects estimates were used. Results were computed by omitting each study (on the left) in turn. The two ends of every broken line represented the 95% confidence interval.
Figure 5.
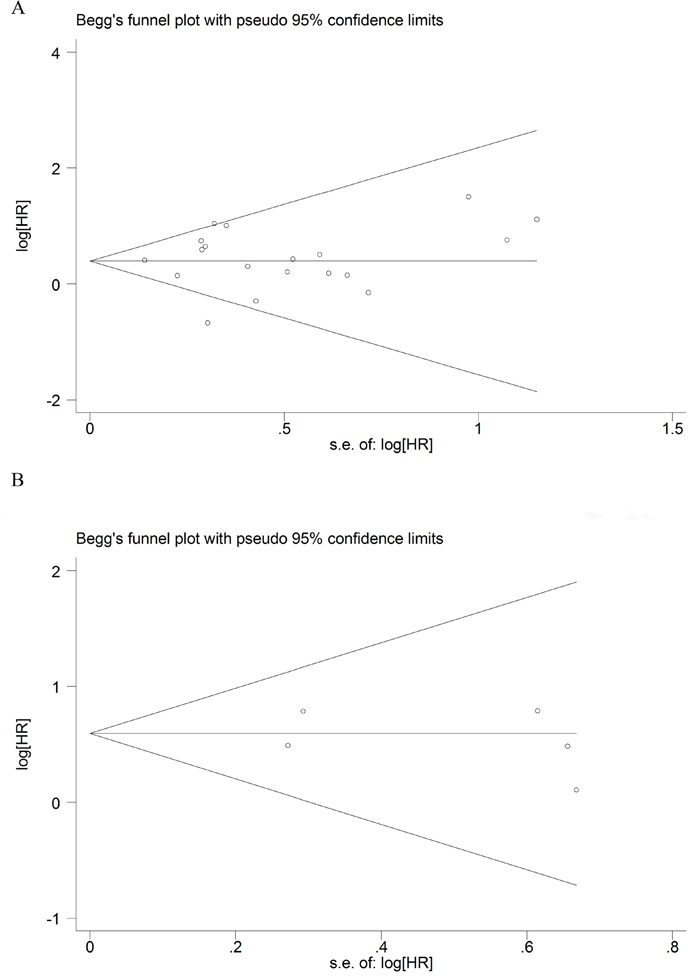
Funnel plot for the assessment of publication bias of the included literature for OS (A) and DFS (B).
Cox-2 expression and clinical parameters
We analyzed the associations between COX-2 expression and different clinical parameters such as age, FIGO stage, histological type, et al. Pooled ORs showed that COX-2 expression correlated with FIGO stage (I/II vs. III/IV), histological type (serous vs. others) and age (young vs. old), while no significant correlations were found between COX-2 expression and tumor grade, lymph node transmission and menopausal status. Then we carried out the sensitivity analysis to evaluate the influence of the single study to the final results, the results did not change in the analysis of FIGO stage, tumor grade, lymph node transmission and menopausal status, while the results changed in the analysis of age and histological type when we deleted one single study (data not show). And there was no publication bias in all of these analysis. The details were showed in Table 3.
Table 3. Association of COX-2 expression with clinical features in ovarian cancer.
| Clinical parameter | Number of studies | OR | 95%CI | P* |
|---|---|---|---|---|
| Age (young vs. old) | 7 | 1.37 | 1-1.81 | 0.126 |
| FIGO stage (I/II vs. III/IV) | 9 | 2.41 | 1.28-4.53 | 0.128 |
| Histological type (serous vs. others) | 7 | 0.69 | 0.48-0.98 | 0.119 |
| Tumor grade (Low 1/2 vs. high 3) | 11 | 1.2 | 0.76-1.89 | 0.858 |
| Lymph node transmission (positive vs. negative) | 3 | 2.36 | 0.78-7.11 | 0.831 |
| Menopausal status (pre vs. post) | 2 | 1.2 | 0.45-3.2 | - |
Abbreviations: OR: odds ratio; CI: confidence interval; *P value for publication bias.
DISCUSSION
COX-2 is the rate-limiting enzyme involved in the conversion of arachidonic acid to various prostaglandins. Studies showed that COX-2 overexpressed in inflammatory and many malignancies [30–32]. The prognostic impact of COX-2 expression had been reported in different tumors [33–36]. Its role in ovarian cancer patients had been reported in several studies, while the results were inconsistent. These conflicting might be affected by the relatively limited sample size included in individual studies. Meta-analysis is an efficient way to combine small studies and get reliable results.
A previous meta-analysis reported the prognostic impact of COX-2 on ovarian cancer mortality by Lee et al [37]. But there are some issues we should concern in their article. First, they mentioned that there were 17 studies included in their analysis, but there were no data for the studies of Sakamoto [38] and Menczer [39]. Second, there were some mistakes in the data extraction for the studies of Ferrero [16] and Ferrandina [28]. Lee extracted the results of univariate analysis while not multivariate from Ferrero. In the study of Ferrandina, there were two Kaplan-Meier plots for different subgroups, while Lee just extracted one of them to represent the total patients. Furthermore, there are some new data available, so, the purpose of this study was to update the meta-analysis and get a more accruable result.
The present meta-analysis, which included 1,867 ovarian patients from 18 studies, indicated that higher COX-2 expression was a signal of poor prognosis for ovarian cancer patients. Our subgroup analysis by follow-up times revealed that the higher expression of COX-2 was strongly associated with poor prognoses regardless the length of the follow-up time. Similarly, we found significant associations between COX-2 overexpression and survival rate in most subgroup analysis. Furthermore, we found there were significant correlations between COX-2 expression and FIGO stage, histological type and the age of patients. Based on these results, COX-2 could be a valuable prognostic biomarker and a therapy target for ovarian cancer patients in clinical trials.
We did not totally understand the exact mechanism by which COX-2 overexpression causes poor prognosis in ovarian cancer patients. COX-2 expression could be induced by various factors, such as mitogens, cytokines, and prostaglandins [40]. It had been shown that COX-2 plays an essential role during tumorigenesis [7, 41]. The overexpression of COX-2 could cause the growth, invasion, migration of ovarian tumor cell, and chemoresistance in ovarian cancers patients [11, 19, 42]. All of these could reduce the survival rate of the patients. More studies are required to investigate the specific molecular mechanism of COX-2 overexpression reducing the survival rate of ovarian cancer patients.
Heterogeneity and publication bias are two important factors to evaluate a meta-analysis. In this study, heterogeneity test revealed there was no obvious heterogeneity in the main analysis and most of the subgroup analysis. The heterogeneity appeared in several subgroups analysis (sample size ≥100 and multivariate analysis). It may partly come from various antibodies, different staining scores or different cut-off values for the higher COX-2 expression level among different studies. So selection of appropriate staining scores and cut-off values would help to improve the reliability of the results and reduce the heterogeneity among different studies. As for publication bias, no proof of publication bias was found. These findings suggested that the results of the meta-analysis were reliable. However, the present study still had some limitations. First, this study is based on literature searching; it is possible that there are some studies were not included in the analysis, even though public bias was not found in this study. Second, most of the enrolled studies just had limited patients, the sample size was small. Third, the number of enrolled studies is relatively small for Asian and North American in the subgroup analysis by study region.
In conclusion, the patients with higher COX-2 expression had a significant poorer OS and DFS. COX-2 expression could be a prognostic marker to help to define high risk patients and find novel therapeutic target for ovarian cancer. But more studies, especially the studies with large number of patients, were needed to clarify the relationships between COX-2 expression and survival rate of ovarian cancer patients.
MATERIALS AND METHODS
Literature searching and study selection
PubMed, Embase, and CNKI were used to retrieve potentially eligible studies published by December 2016. The keywords for the search in these databases included: “ovarian cancer” or “ovarian carcinoma” or “ovarian tumor” or “ovarian tumour” or “ovarian neoplasm” or “ovarian malignancy”, “COX-2” or “Cyclooxygenase-2”, and “prognosis” or “survival” or “outcome” or “mortality”. In addition, we also conducted the manual searches of references in all eligible studies to identify potential missing publications. There were no any restrictions on the searches.
Studies would not be considered unless they met the following criteria: provided survival data in ovarian cancer patients stratified by COX-2 expression and sufficient data to calculate an estimate of hazard ratio (HR) and a 95% confidence interval (CI); all selected ovarian cancer patients were pathologically confirmed and the protein expression of COX-2 was measured in the ovarian cancer specimen. All articles were reviewed to avoid the duplicate data. The most recent or most complete publication was enrolled if there were overlaps between studies.
Data extraction and quality assessment
Two investigators conducted the literature review and extracted data by using a standardized data extraction form, separately. For each article, the following information was extracted: first authors and the year of publication, sample size, country, FIGO stage, histological subtype, follow-up time, COX-2 detection methods, cut-off level of the higher expression, number of high expression patients, HR and 95% CI and methods of HR estimation. For the studies which provided both the univariate and multivariate analysis, HR and 95% CI of multivariate analysis were extracted because they could avoid the interference of confounding factors. If the results of survival analysis were not reported by the authors, we extracted the survival data from the Kaplan-Meier curves and calculated HR and 95% CI by the methods of Jayne et al [43]. For the studies which provided the relationships between COX-2 expression and clinical parameters, we extracted the related data. We used the New-castle-Ottawa Scale (NOS) to evaluate the quality of each study [44]. Studies were assigned to high quality group if they had the score of 7 or higher. The extracted data were crosschecked and disagreements were resolved by discussion.
Statistical analysis
The two outcomes endpoints were overall survival (OS) and disease-free survival (DFS). Pooled HRs with 95% CIs were calculated based on random-effects model due to the possible substantial heterogeneity among studies [45]. In order to evaluate the relationships between COX-2 expression and clinical parameters, pooled ORs and 95% CIs were calculated based on random-effects model too. Heterogeneity was examined by I2 statistic, I2 ≥ 50% indicated the presence of significant heterogeneity [46]. We further investigated potential heterogeneity by subgroup analyses stratified by study quality, sample size, follow-up time, histology subtypes, region of the study, and calculation methods of HR. Sensitivity analysis was conducted to evaluate the stability of the results. Begger's funnel plots and Egger's test were used to find the potential publication bias [47, 48]. All statistical analyses were performed by Stata statistical software (version 11.0; Stata Corporation, College Station, TX, USA).
Footnotes
Author contributions
H.S., Y.Q. and Y.J. conceived and designed the research. X.Z. and D.S. collected articles and extracted data. X.J. and L.X. performed the statistical analysis. H.S. and Y.J. wrote the manuscript. All of the authors read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
FUNDING
This work was supported by the grant from China Scholarship Council (201608230035) and National Natural Science Foundation of China (81371617).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Fishman DA, Bozorgi K. The scientific basis of early detection of epithelial ovarian cancer: the National Ovarian Cancer Early Detection Program (NOCEDP) Cancer Treat Res. 2002;107:3–28. doi: 10.1007/978-1-4757-3587-1_1. [DOI] [PubMed] [Google Scholar]
- 4.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Stolina M, Yang SC, Baratelli F, Lin JF, Atianzar K, Luo J, Zhu L, Lin Y, Huang M, Dohadwala M, Batra RK, Dubinett SM. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res. 2003;9:961–968. [PubMed] [Google Scholar]
- 7.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 8.Wu QB, Sun GP. Expression of COX-2 and HER-2 in colorectal cancer and their correlation. World J Gastroenterol. 2015;21:6206–6214. doi: 10.3748/wjg.v21.i20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokouchi H, Kanazawa K. Revisiting the role of COX-2 inhibitor for non-small cell lung cancer. Transl Lung Cancer Res. 2015;4:660–664. doi: 10.3978/j.issn.2218-6751.2015.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang CM, Kim HK, Kim H, Lee WJ. Cyclooxygenase-2 (COX-2) expression in solid pseudopapillary tumor of the pancreas: a pilot study. Pancreas. 2011;40:159–161. doi: 10.1097/MPA.0b013e3181f74ca5. [DOI] [PubMed] [Google Scholar]
- 11.Gu P, Su Y, Guo S, Teng L, Xu Y, Qi J, Gong H, Cai Y. Over-expression of COX-2 induces human ovarian cancer cells (CAOV-3) viability, migration and proliferation in association with PI3-k/Akt activation. Cancer Invest. 2008;26:822–829. doi: 10.1080/07357900801941860. [DOI] [PubMed] [Google Scholar]
- 12.Ali-Fehmi R, Semaan A, Sethi S, Arabi H, Bandyopadhyay S, Hussein YR, Diamond MP, Saed G, Morris RT, Munkarah AR. Molecular typing of epithelial ovarian carcinomas using inflammatory markers. Cancer. 2011;117:301–309. doi: 10.1002/cncr.25588. [DOI] [PubMed] [Google Scholar]
- 13.Denkert C, Kobel M, Pest S, Koch I, Berger S, Schwabe M, Siegert A, Reles A, Klosterhalfen B, Hauptmann S. Expression of cyclooxygenase 2 is an independent prognostic factor in human ovarian carcinoma. Am J Pathol. 2002;160:893–903. doi: 10.1016/S0002-9440(10)64912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo SS, Song YS, Kang DH, Park IA, Bang YJ, Kang SB, Lee HP. Expression of cyclooxygenase-2 in association with clinicopathological prognostic factors and molecular markers in epithelial ovarian cancer. Gynecol Oncol. 2004;92:927–935. doi: 10.1016/j.ygyno.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 15.Raspollini MR, Amunni G, Villanucci A, Boddi V, Baroni G, Taddei A, Taddei GL. Expression of inducible nitric oxide synthase and cyclooxygenase-2 in ovarian cancer: correlation with clinical outcome. Gynecol Oncol. 2004;92:806–812. doi: 10.1016/j.ygyno.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Ferrero A, Dompe D, Ravarino N, Ramella A, Fuso L, Maggiorotto F, Tripodi E, Zola P. Angiogenesis and molecular markers in advanced epithelial ovarian cancer: a retrospective study. Gynecol Oncol. 2011;123:301–307. doi: 10.1016/j.ygyno.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Surowiak P, Materna V, Denkert C, Kaplenko I, Spaczynski M, Dietel M, Zabel M, Lage H. Significance of cyclooxygenase 2 and MDR1/P-glycoprotein coexpression in ovarian cancers. Cancer Lett. 2006;235:272–280. doi: 10.1016/j.canlet.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 18.Wang M, He Y, Shi L, Shi C. Multivariate analysis by Cox proportional hazard model on prognosis of patient with epithelial ovarian cancer. Eur J Gynaecol Oncol. 2011;32:171–177. [PubMed] [Google Scholar]
- 19.Athanassiadou P, Grapsa D, Athanassiades P, Gonidi M, Athanassiadou AM, Tsipis A, Patsouris E. The prognostic significance of COX-2 and survivin expression in ovarian cancer. Pathol Res Pract. 2008;204:241–249. doi: 10.1016/j.prp.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Erkinheimo TL, Lassus H, Finne P, van Rees BP, Leminen A, Ylikorkala O, Haglund C, Butzow R, Ristimaki A. Elevated cyclooxygenase-2 expression is associated with altered expression of p53 and SMAD4, amplification of HER-2/neu, and poor outcome in serous ovarian carcinoma. Clin Cancer Res. 2004;10:538–545. doi: 10.1158/1078-0432.ccr-0132-03. [DOI] [PubMed] [Google Scholar]
- 21.Khalifeh I, Munkarah AR, Lonardo F, Malone JM, Morris R, Lawrence WD, Ali-Fehmi R. Expression of Cox-2, CD34, Bcl-2, and p53 and survival in patients with primary peritoneal serous carcinoma and primary ovarian serous carcinoma. Int J Gynecol Pathol. 2004;23:162–169. doi: 10.1097/00004347-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Steffensen KD, Waldstrom M, Jeppesen U, Jakobsen E, Brandslund I, Jakobsen A. The prognostic importance of cyclooxygenase 2 and HER2 expression in epithelial ovarian cancer. Int J Gynecol Cancer. 2007;17:798–807. doi: 10.1111/j.1525-1438.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- 23.Magnowska M, Zaborowski M, Surowiak P, Nowak-Markwitz E, Zabel M, Spaczynski M. COX-2 expression pattern is related to ovarian cancer differentiation and prognosis, but is not consistent with new model of pathogenesis. Ginekol Pol. 2014;85:335–341. doi: 10.17772/gp/1733. [DOI] [PubMed] [Google Scholar]
- 24.Taskin S, Dunder I, Erol E, Taskin EA, Kiremitci S, Oztuna D, Sertcelik A. Roles of E-cadherin and cyclooxygenase enzymes in predicting different survival patterns of optimally cytoreduced serous ovarian cancer patients. Asian Pac J Cancer Prev. 2012;13:5715–5719. [PubMed] [Google Scholar]
- 25.Lou WZ, Shen K, Zhang Y, Lang JH. [Relationship of cyclooxygenase 2 expression and chemotherapy response and prognosis in human ovarian carcinoma] [Article in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2004;39:529–532. [PubMed] [Google Scholar]
- 26.Lee JS, Choi YD, Lee JH, Nam JH, Choi C, Lee MC, Park CS, Juhng SW, Min KW. Expression of cyclooxygenase-2 in epithelial ovarian tumors and its relation to vascular endothelial growth factor and p53 expression. Int J Gynecol Cancer. 2006;16:247–253. doi: 10.1111/j.1525-1438.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto J, Toyoki H, Sakaguchi H, Jahan I, Alam SM, Tamaya T. Clinical implications of expression of cyclooxygenase-2 related to angiogenesis in ovarian cancer. Oncol Rep. 2006;15:21–25. [PubMed] [Google Scholar]
- 28.Ferrandina G, Lauriola L, Zannoni GF, Fagotti A, Fanfani F, Legge F, Maggiano N, Gessi M, Mancuso S, Ranelletti FO, Scambia G. Increased cyclooxygenase-2 (COX-2) expression is associated with chemotherapy resistance and outcome in ovarian cancer patients. Ann Oncol. 2002;13:1205–1211. doi: 10.1093/annonc/mdf207. [DOI] [PubMed] [Google Scholar]
- 29.Ozuysal S, Bilgin T, Ozgur T, Celik N, Evrensel T. Expression of cyclooxygenase-2 in ovarian serous carcinoma: correlation with angiogenesis, nm23 expression and survival. Eur J Gynaecol Oncol. 2009;30:640–645. [PubMed] [Google Scholar]
- 30.Lee JW, Park JH, Suh JH, Nam KH, Choe JY, Jung HY, Chae JY, Moon KC. Cyclooxygenase-2 expression and its prognostic significance in clear cell renal cell carcinoma. Korean J Pathol. 2012;46:237–245. doi: 10.4132/KoreanJPathol.2012.46.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uefuji K, Ichikura T, Mochizuki H. Increased expression of interleukin-1alpha and cyclooxygenase-2 in human gastric cancer: a possible role in tumor progression. Anticancer Res. 2005;25:3225–3230. [PubMed] [Google Scholar]
- 32.Barisik NO, Keser SH, Gul AE, Sensu S, Kandemir NO, Kucuk HF, Gumus M, Karadayi N. The value of COX-2 expression in the prognostic parameters of invasive ductal carcinoma of the breast. Med Oncol. 2011;28:703–708. doi: 10.1007/s12032-010-9503-6. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Wu F, Pei HL, Gu WD, Ning ZH, Shao YJ, Huang J. Analysis of the correlation between P53 and Cox-2 expression and prognosis in esophageal cancer. Oncol Lett. 2015;10:2197–2203. doi: 10.3892/ol.2015.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes MD, Chen WY, Schnitt SJ, Collins L, Colditz GA, Hankinson SE, Tamimi RM. COX-2 expression predicts worse breast cancer prognosis and does not modify the association with aspirin. Breast Cancer Res Treat. 2011;130:657–662. doi: 10.1007/s10549-011-1651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsubayashi H, Infante JR, Winter J, Klein AP, Schulick R, Hruban R, Visvanathan K, Goggins M. Tumor COX-2 expression and prognosis of patients with resectable pancreatic cancer. Cancer Biol Ther. 2007;6:1569–1575. doi: 10.4161/cbt.6.10.4711. [DOI] [PubMed] [Google Scholar]
- 36.Schildberg C, Abbas M, Merkel S, Agaimy A, Dimmler A, Schlabrakowski A, Croner R, Leupolt J, Hohenberger W, Allgayer H. COX-2, TFF1, and Src define better prognosis in young patients with gastric cancer. J Surg Oncol. 2013;108:409–413. doi: 10.1002/jso.23416. [DOI] [PubMed] [Google Scholar]
- 37.Lee JY, Myung SK, Song YS. Prognostic role of cyclooxygenase-2 in epithelial ovarian cancer: a meta-analysis of observational studies. Gynecol Oncol. 2013;129:613–619. doi: 10.1016/j.ygyno.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Sakamoto A, Yokoyama Y, Umemoto M, Futagami M, Sakamoto T, Bing X, Mizunuma H. Clinical implication of expression of cyclooxygenase-2 and peroxisome proliferator activated-receptor gamma in epithelial ovarian tumours. Br J Cancer. 2004;91:633–638. doi: 10.1038/sj.bjc.6602009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menczer J, Schreiber L, Kravtsov V, Berger E, Golan A, Levy T. Cox-2 immunohistochemical expression in epithelial ovarian carcinoma and platin sensitivity. Eur J Gynaecol Oncol. 2009;30:531–535. [PubMed] [Google Scholar]
- 40.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 41.Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18:3831–3852. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- 42.Uddin S, Ahmed M, Hussain A, Assad L, Al-Dayel F, Bavi P, Al-Kuraya KS, Munkarah A. Cyclooxygenase-2 inhibition inhibits PI3K/AKT kinase activity in epithelial ovarian cancer. Int J Cancer. 2010;126:382–394. doi: 10.1002/ijc.24757. [DOI] [PubMed] [Google Scholar]
- 43.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 45.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 46.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 47.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 48.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]


