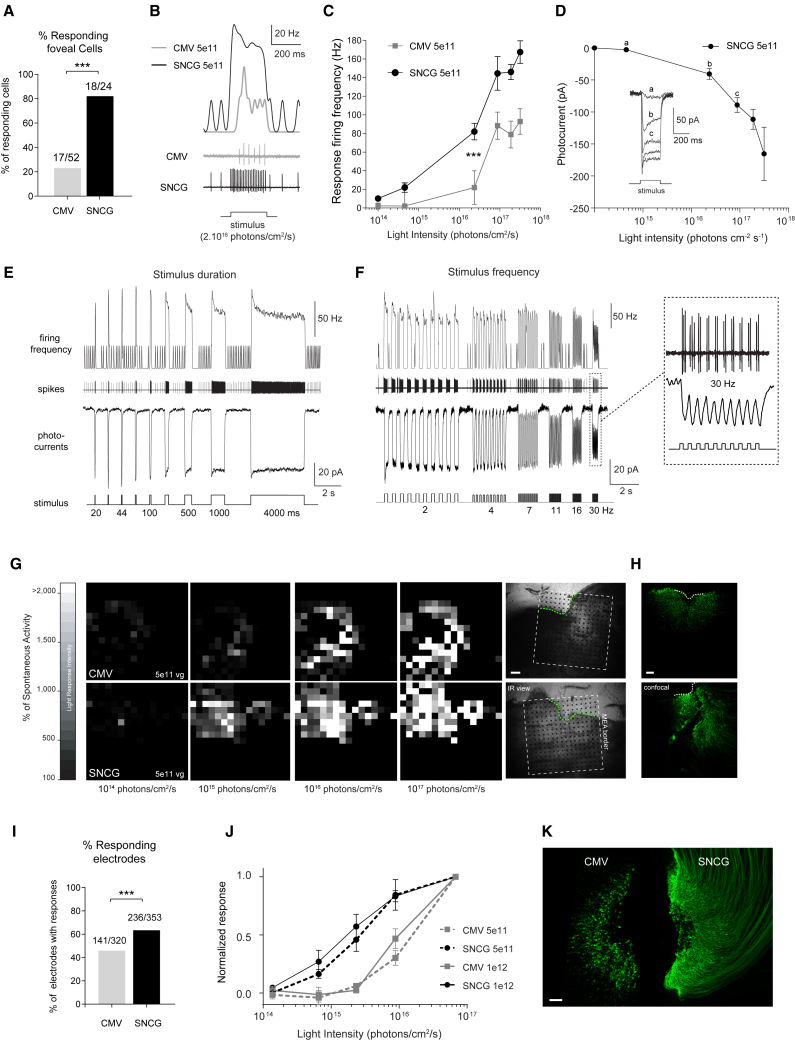
Figure 5.
Characteristics of CatCh-Mediated Light Responses at 6 Months Post-Injection
(A–F) Results from single-cell recordings (using cell-attached and whole-cell patch-clamp configurations) of ganglion cells recorded in the peri-foveolar region of retinas injected with either CMV-CatCh or SNCG-CatCh at a dose of 5 × 1011 vg, after application of L-AP4. (A) Comparison of the proportions of responsive cells recorded either from CMV-CatCh or SNCG-CatCh. (B) Representative recordings showing the spiking frequency obtained for SNCG-CatCh compared to CMV-CatCh (bottom, raw data; top, rate evolution curves) during light stimulation (intensity of 2 × 1016 photons/cm2/s, wavelength of 480 nm). (C) Response firing frequency of RGCs as a function of light stimulation intensity for CMV-CatCh and SNCG-CatCh at the 5 × 1011 vg viral dose. (D) CatCh photocurrents (inward currents recorded at −60 mV in whole-cell configuration) of RGCs as a function of light stimulation intensity for SNCG-CatCh at 5× 1011 vg per eye. Photocurrents of a representative cell are shown under the intensity curve. (E) Response characteristics to varying stimulus duration. The middle and top rows show raw data (cell-attached) of the spiking activity and firing frequency in response to stimuli of increasing duration (20 ms to 4 s). The bottom row shows the photocurrent recorded in the whole-cell configuration from another representative cell using the same pattern of stimulation. (F) Response characteristics to varying stimulation frequencies. The middle and top rows show raw data (cell-attached) showing the spiking activity of a cell and its firing frequency in response to stimuli of increasing frequencies (2–30 Hz). The bottom row shows photocurrents recorded in another representative cell using the same pattern of stimulation. A magnified trace is shown at 30 Hz displaying the robust photocurrents and spiking activity obtained for each of the 10 successive stimulations. (G–K) Results from MEA recordings and histology. (G) Grayscale maps based on firing rates of responding neurons (expressed as a percentage of their spontaneous activity) at increasing light intensities in primate retinas injected with AAV2-SNCG-hCatCh-GFP (top) and AAV2-SNCG-hCatCh (bottom) at 5 × 1011 vg per eye, after application of L-AP4. On the right, the MEA chip perimeter is represented by a dotted white square, and the putative fovea area is indicated by a dashed green curves in the pictures. The black dots represent the locations of the MEA recording electrodes. The scale bar represents 200 μm. (H) The same foveal regions after MEA recordings and RGC immunolabeling. The retinal flat mount was stained with antibodies against channelrhodopsin (green). Note that the SNCG retina was partially damaged while handling the tissue after MEA procedures. The scale bar represents 100 μm. (I) Percentage of responsive MEA electrodes for each promoter at 5 × 1011 vg per eye. (J) Normalized responses of RGCs to light at increasing intensities at 6 months post-injection. Each line represents discharge frequencies normalized across retinas for the high-dose (n = 2 retinas) and low-dose (n = 3 retinas) groups. (K) Foveal regions of two representative NHP retinas that received a 1012 vg dose of AAV-CatCh expressed under the CMV (left) or SNCG (right) promoter, after MEA recordings and immunolabeling. The retinal flat mount was stained with antibodies against channelrhodopsin (green). The scale bar represents 100 μm. Error bars represent SEMs. ***p < 0.001, statistically significant.