Figure 4.
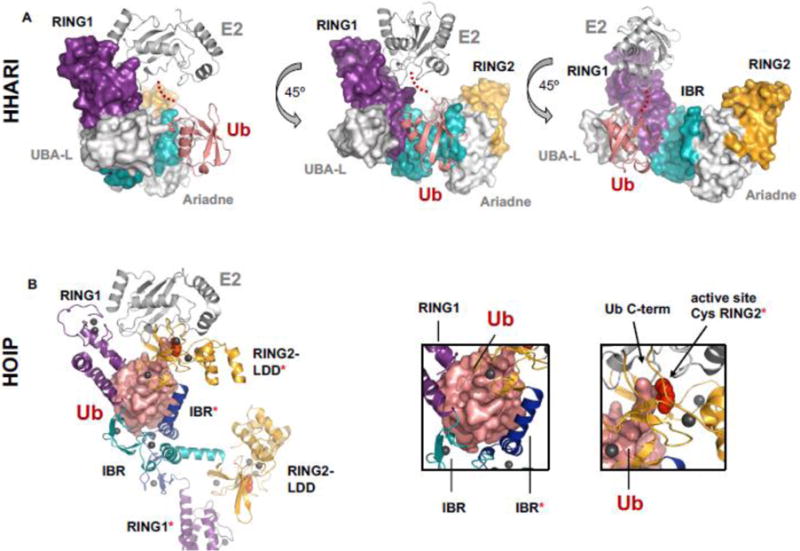
Comparison of an auto-inhibited RBR/E2~Ub complex and an “activated” RBR/E2~Ub complex. A) HHARI bound to E2~Ub (E2 = UbcH7; PDB 5UDH) is still auto-inhibited. Ub (salmon cartoon) makes no contacts with any domains of its cognate HHARI molecule. In the auto-inhibited state the active-site Cys (red spheres, but not visible in the surface representation of the auto-inhibited structure) is occluded by the Ariadne domain and therefore not visible in the surface representation shown here. RING1 (purple surface) and RING2 (orange surface) domains are far apart (as seen in apo-HHARI, Fig. 2A). IBR is shown in cyan surface representation. Density for the C-terminus of Ub is missing in the crystal structure and is instead indicated by red dots. B) HOIP RBR module bound to E2~Ub (E2 = UbcH5; PDB 5EDV). The Ub moiety (salmon surface) of E2~Ub contacts IBR and RING2 domains of two different HOIP molecules (left box, all domains belonging to the second (non-cognate) polypeptide are marked with a [*]). Domain colors are the same as A). The domain swap in the crystal affords a view of what an activated version of HOIP RBR could look like, with the C-term of ~Ub conjugated to the E2 (right box, black arrow) in proximity to the active-site Cys of HOIP (right box; black arrow and red spheres).
