Abstract
Background/Objectives
Processed foods are considered major contributors to the worldwide obesity epidemic. In addition to high sugar and fat contents, processed foods contain large amounts of salt. Due to correlations with rising adiposity, salt has recently been proposed to be obesogenic. This study investigated three hypotheses: i) high salt contributes to weight gain and adiposity in juvenile female rats, ii) puberty onset would be altered because salt is known to affect neuronal systems involved in activating the reproductive system, and iii) enhanced adiposity will act synergistically with salt to drive early puberty onset.
Design
Female weanling rats (post-natal day 21, n=105) were fed a low fat/low salt diet, low fat/high salt diet, high fat/low salt diet, or a high salt/high fat diet for 24 days. Metabolic measures, including weight gain, food intake, fecal output, activity, and temperature were recorded in subsets of animals.
Results
Body weight, retroperitoneal and perirenal fat pad weight, and adipocyte size were all lower in animals fed high fat/high salt compared to animals fed high fat alone. Leptin levels were reduced in high fat/high salt fed animals compared to high fat/low salt fed animals. Daily calorie intake was higher initially but declined with adjusted food intake and was not different among groups after 5 days. Osmolality and corticosterone were not different among groups. Fecal analysis showed excess fat excretion and a decreased digestive efficiency in animals fed high fat/low salt but not in animals fed high fat/high salt. Although respiratory exchange ratio was reduced by high dietary fat or salt, aerobic resting metabolic rate was not affected by diet. High salt delayed puberty onset, regardless of dietary fat content.
Conclusions
Salt delays puberty and prevents the obesogenic effect of a high fat diet. The reduced weight gain evident in high salt fed animals is not due to differences in food intake or digestive efficiency.
Introduction
Childhood obesity is arguably the most important international public health challenge of the 21st century. An increased prevalence of childhood obesity has been observed worldwide, in both developed and developing countries1. In the 1960s, an estimated 4.2% of 6–11 year olds (1 in 24) and 4.6% of 12–19 year olds (1 in 22) in the US were obese2. By 2012, this had increased to 17.7% of 6–11 year olds (1 in 6) and 20.5% of 12–19 year olds (1 in 5)3. Being overweight or obese in childhood tends to persist into adulthood4: 55% of obese children are obese in adolescence, 80% of obese adolescents are still obese in adulthood, and 70% will be obese over age 30. Childhood and adolescent obesity is associated with a number of medical complications: hypertension, type 2 diabetes, coronary heart disease, stroke and several types of cancer5–7. Public health researchers and clinicians concur that prevention could be key to controlling the obesity epidemic. The etiology of this epidemic is multifactorial, including excessive calorie intake8, a sedentary lifestyle9, genetics10, as well as socioeconomic elements, such as lack of access to unprocessed food. The high fat and sugar content in processed foods are implicated in increased adiposity11 and recently it has been hypothesized that salt may play a critical role in the obesity epidemic. A combination of high salt and high fat has been associated with an increased risk of higher BMI, waist circumference, and subcutaneous abdominal adipose tissue12–14. Preclinical research is mixed, with a majority15–17 indicating an association between salt intake and body weight and/or adiposity, but more recently, this has been contested, at least in mice18, 19. However, all previously reported studies have been on males and extended beyond the adolescent period. Extrapolating findings in the male to the female is problematic20. Thus, a primary objective of our study was to determine the effect of dietary salt on adolescent female rats fed either a normal or high fat diet. Based on the earlier rat studies15–17, we hypothesized that salt would increase adiposity, especially in rats fed a high fat diet.
It has been hypothesized that increased adiposity, or an increased rate of change in BMI, are inductive of puberty in girls21, 22. Although the age at menarche differs across countries, there is agreement that the age of puberty has been decreasing. In the past century, puberty has advanced almost 2 years in the US23, 24 and a year earlier in Denmark25. Early puberty is not innocuous: it has been linked to a higher risk of psychological problems, risk-taking behavior, future breast cancer and, recently, the development of adolescent polycystic ovarian syndrome26–29. As we postulated that salt would be adipogenic, we further hypothesized female rats on a high salt diet would commence puberty earlier. However, several longitudinal studies have reported that BMI does not explain all cases of advanced puberty in girls24, 30. As salt activates the neurokinin B (NKB) system in rats31 and NKB plays an important role in puberty onset32, we further hypothesized that rats on a high salt, but low fat, diet would similarly exhibit advanced puberty.
Our overall unifying hypothesis was that salt would increase adiposity, especially in high fat dietary conditions, and that the synergistic effects would significantly advance puberty in animals fed a diet high in both salt and fat.
Materials and Methods
Animals
Female Sprague-Dawley rats (n = 105; Charles River, Wilmington, MA) were kept under controlled conditions on a 12:12 light:dark cycle (21°C). To standardize competition for food and maternal attention, litters were reduced to 10–12 pups. At weaning (postnatal day (pnd) 21), rats were placed on specially formulated diets (Research Diets, New Brunswick, NJ). As weaning weight is known to affect age at puberty33, only rats with weights within ±2 SD of the mean were used. Animals were weighed daily and monitored for vaginal opening from pnd 25. Group size was estimated using data from the effect of a high fat diet on puberty onset34. Animals were euthanized on pnd 45, plasma collected and organ weights recorded. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Wyoming.
Body weight experiment: 53 animals were housed 3–6 per cage for the initial weight study.
Food Intake experiments: 24 animals were placed in individual cages for daily food intake.
Alternate diet experiments: 29 animals were placed in individual cages for daily food intake.
Fecal analysis and respiratory exchange ratio experiment: 28 rats were placed in individual metabolic cages that allowed for the collection of urine, fecal matter and recording of daily food intake.
Diets
Animals were randomly assigned to a control low fat/low salt (LFLS; 10% kcal fat, 0.3% salt) or low fat/high salt (LFHS; 10% kcal fat, 8.3% salt), high fat/low salt (HFLS; 60% kcal fat, 0.3% salt), or high fat/high salt (HFHS; 60% kcal fat, 8.3% salt;) diet (Supplemental Table 1). In the alternate diet experiment, diets alternated with the control LFLS diet. Food and tap water were available ad libitum. Diets using 8% salt are routinely used in hypertension studies35, 36. Moreover, previous studies have reported an increase in body weight on an 8% salt diet37, 38. A 60% high fat diet is known to induce obesity in rodents and, importantly, does not discriminate between obesity prone and obesity resistant animals39. As diets were colored, investigators were not blinded to the treatments.
Leptin and Corticosterone Assays and Osmolality Analysis
Plasma leptin concentrations were determined in triplicate by a commercially available enzyme linked immunosorbent assay (ELISA) kit (mouse/rat leptin ELISA, BioVendor, Asheville, NC). Plasma corticosterone concentrations were determined in triplicate using a commercially available ELISA (Enzo Life Sciences, Farmingdale, NY). The detection limits were 50 pg/ml and 1.4 pg/ml, respectively. Plasma osmolality (Multi-Osmette, Precision Systems) was determined in plasma for 4 rats per diet in the food intake experiment. Hematocrit was determined in 7 rats per diet.
Adipocyte Size
Adipocyte size was measured in 17 animals randomly selected from the body weight experiment. Retroperitoneal fat was fixed in formalin and embedded in paraffin. Sections (40 µm) were cut on a microtome, mounted on slides, deparaffinized in xylene, rehydrated, and stained with hematoxylin and eosin. Cells were photographed on a Nikon Eclipse 3800 and adipocyte size was measured with the ImageJ plugin Adiposoft (http://fiji.sc/Adiposoft). Six sections were used from each animal, with every whole cell within that section recorded. Adipocytes were averaged to provide a mean for each animal.
Fecal Analysis
A recent study18 reported that salt impacted the digestive efficiency of lipids. Thus, we performed 3 independent assays of fecal lipid content including the steatocrit used in the previous study18.
Feces were collected daily across the duration of the experiment. Lipids were extracted by pulverization with a mortar and pestle, and 0.5g was vortexed for 24h with a chloroform: methanol: water (1:2:8) solution. Chloroform, 1M KCl and 0.15M HCl were added to the mixture, vortexed and centrifuged. The top aqueous layer was siphoned off and the chloroform phase was filtered with 0.45µm pore cellulose acetate filters (Whatman, Maidstone, UK) to remove solid particles. The chloroform was evaporated off and the remaining lipids weighed.
For the fecal acid steatocrit analysis, 50g of pulverized freeze-dried feces were dissolved in 200µl of freshly prepared 1N perchloric acid to which 100µl of 0.5% Oil-Red-O was added. The resulting slurry was loaded into capillary tubes and centrifuged (10 min; 10000g). Tubes were digitally photographed and the proportion of red-stained oil layer to the total sample length was calculated using ImageJ.
Bomb calorimetry was used to estimate the excreted calories. Feces were pulverized and pelletized before analysis in a 6200 Isoperibol Calorimeter (Parr Instrument Company, Moline, IL). By calculating calories absorbed (digestive efficiency) using food intake and fecal calorimetry data, we were able to examining how a daily intake of calories influenced daily weight gain (energy efficiency).
Respiratory Exchange Ratio (RER) and Resting Metabolic Rate (RMR)
To obtain a measure of substrate utilization, RER was obtained from 28 rats on pnd29 and pnd38. An RER of 1.0 indicates pure carbohydrate use, whereas an RER of 0.7 indicates almost pure fat metabolism. RER was measured using a custom-made single-chamber system paired with a FoxBox Respirometry System (Sable Systems, Las Vegas, NV), which automatically corrects for barometric pressure. Resting O2 and CO2 data were obtained after a stable baseline was recorded. O2 and CO2 levels were recorded every minute for 5 min and RER was calculated from the 5 min average. Data from one LFHS animal was excluded from analysis as it was outside of 5 SDs of the group mean.
Aerobic metabolic rate (RMR) was estimated from RER using the equation derived from Lusk40
It should be noted that aerobic RMR underestimates actual RMR by 10–12%41.
Body Temperature and Locomotor Activity
On pnd27, body temperature was measured by implanting wax-coated, sterile Thermocron iButtons (Maxim Integrated, San Jose, CA) into the intraperitoneal cavity of 16 rats on LFLS, LFHS, or HFLS diets. Under aseptic conditions, a lateral paramedian incision was made in anesthetized (15:2, ketamine: xylazine; 850µl/kg) rats and a coated ibutton inserted. After suturing, rats were kept on a warm pad until recovery. The incision was coated with a triple antibiotic (Perrigo, Ireland) and the sutured area sprayed with a taste deterrent (Grannick’s Bitter Apple Company, Norwalk, CT) to minimize suture removal. Body temperature was recorded from pnd27 to pnd45. Due to failure of some ibuttons, only 12 rats were used in data analysis.
Locomotor activity was measured at five different time points: pnd21–22, 24–25, 29–33 and 37–38. Activity was measured in digiscan animal activity monitors (Omnitech, Columbus, OH) as the number of beam breaks every 15 min. As rats are nocturnal, they were placed into locomotor boxes at 18:30 and removed the following day at 09:00.
Data analysis
Rats from all four groups (LFLS, LFHS, HFLS, HFHS) from the body weight, food intake, and fecal analysis and respiratory exchange ratio experiments were pooled together for body weight, fad pad, and puberty analyses (n=105). Food intake was normalized to body weight. As an increase in total body weight does not differentiate between growth and/or adiposity increases, tibia length was used to normalize organ weights42. Data were analyzed using Prism 7.0 (Graph Pad Software, La Jolla, CA). Two-way ANOVA with repeated measures was used to determine whether diet influenced body weight over time. Two-way ANOVA was used to determine if there was an interaction between salt and fat with Fishers Least Significant Difference used for post-hoc analysis. A priori comparisons were: LFLS vs LFHS, LFLS vs HFLS, LFHS vs HFHS, and HFLS vs HFHS; these groups were analyzed because these two groups differed by a single dietary factor. Puberty was analyzed by survival curve with a log-rank (Mantel-Cox) test. Treatment effects on RMR were analyzed by ANCOVA (JMP Pro, SAS Institute Inc, Cary, NC) to account for the observed body weight differences among groups. All tests were two-tailed, with significance accepted at p<0.05.
Results
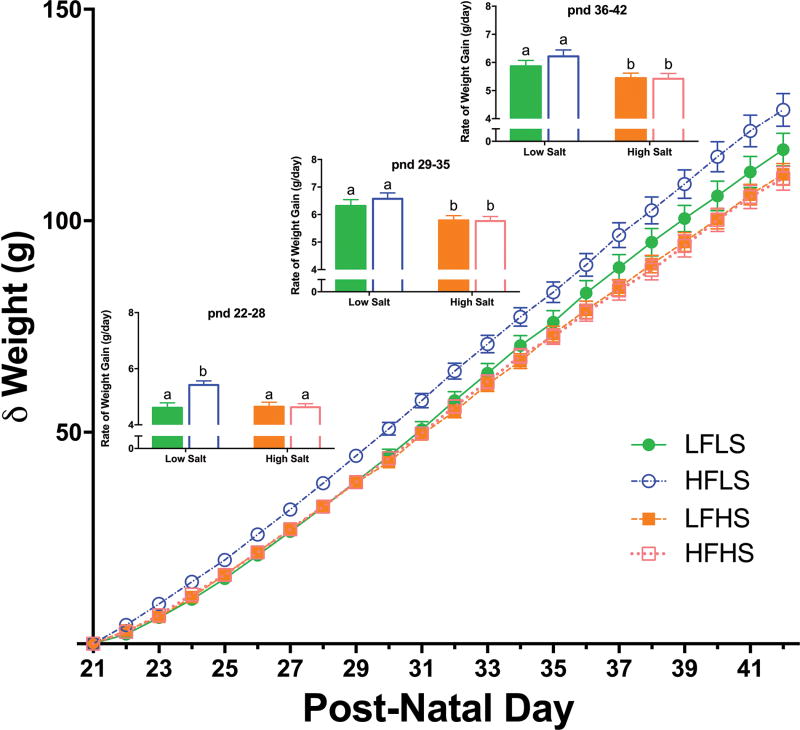
Salt did not affect body weight gain in animals on the low fat diet, but significantly (p<0.001) attenuated the increase in body weight induced by the high fat diet (Figure 1). Body weight in HFLS rats was already significantly different from all groups after only 10 days (p<0.05). This difference between the HFLS rats and other groups persisted until the end of the study. After 24 days, HFLS animals weighed 7% more than LFLS animals and 11% more than HFHS animals. The rate of growth during the first 7 days was higher in HFLS animals (p<0.001), and these rats continued to have increased growth rates compared to the HFHS group (p<0.01). Neither the BMI nor Lee index43 differed among groups. An adjustment to the diets was evident on the first day; calorie intake was more variable and HFLS rats consumed more, and LFHS rats less, than other groups. By 5 days however, animals on low fat diets consumed more food (p<0.05) than animals on high fat diets. Due to this difference in food intake, calorie intake was highest in the HFLS rats for the first 5 days but thereafter there was no difference among groups (p<0.001; Figure 2A). When experimental diets were alternated with the control LFLS diet, there were no differences in food or calorie intake among groups (Supplemental Figure).
Figure 1.
Change in weight relative to starting weight and rate of daily weight gain (inserts) during each week of the study. Weight gain in the HFLS-fed group was significantly greater than all other groups by pnd31. In the first week of the study (pnd 22–28), the rate of daily weight gain in the HFLS rats was significantly higher than LFLS and HFHS groups. The rate of daily weight gain of the HFLS rats was also significantly higher than the HFHS group during the second and third weeks of the study (pnd 29–35 and 36–42). Solid bars = Low Fat; Empty bars = High Fat. Differing letters indicate significant differences of at least p<0.05.
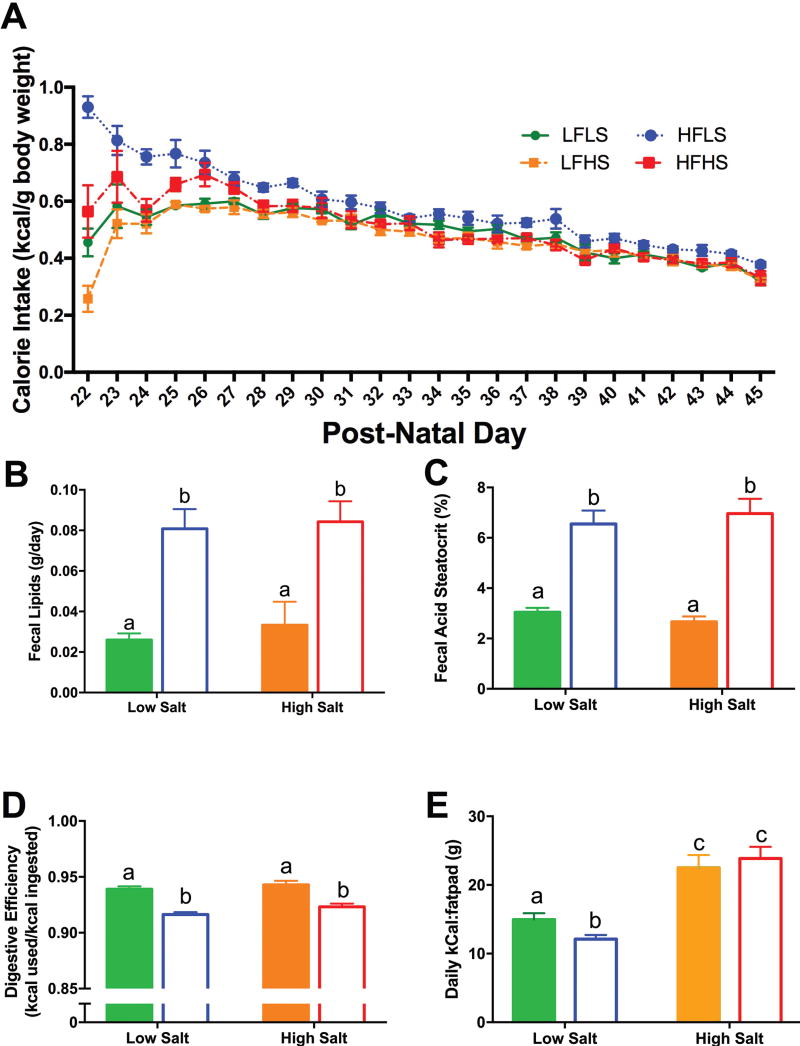
Figure 2.
(A): Calorie intake was higher by the HFLS rats for the first 5 days after which calorie intake was not significantly different among groups. (B): Fecal lipid excretion in both HFLS and HFHS-fed rats was significantly higher than from rats on low fat diets. (C): Fat present in the feces, as measured by fecal steatocrit, was significantly higher in groups fed high fat. (D): Rats fed high fat diets had lower digestive efficiency (calories used relative to calories ingested) compared to those on low fat diets. There was no effect of salt. (E): Deposition of fat when animals were on high salt diets required great calorie consumption. Solid bars = Low Fat; Empty bars = High Fat. Differing letters indicate significant differences of at least p<0.05.
High dietary fat increased fecal lipid excretion (F(1,24)=11.76, p<0.01), and this was not affected by dietary salt (Figure 2B). The fecal steatocrit analysis corroborated the lipid extraction data: there was a significant effect of fat (F(1,24)=87.29, p<0.0001), with significantly more fat in the feces in both HF groups compared to HS groups (p<0.0001 for both; Figure 2C). There was a significant effect of fat on digestive efficiency (F(1,24)=55.8, p<0.0001), with both HF groups showing decreased digestive efficiency compared to LF animals (p<0.0001 for both; Figure 2D) and no effect of high salt. Although total energy efficiency (weight gained from pnd21 to pnd42/total calories absorbed) was not significantly different, the ratio of kCal consumed to gram of fat pad weight revealed that fewer calories were required on a HFLS diet to deposit fat. In contrast, both HS diets required significantly more calories ((F(1,47)=48.06, p<0.0001), Figure 2E).
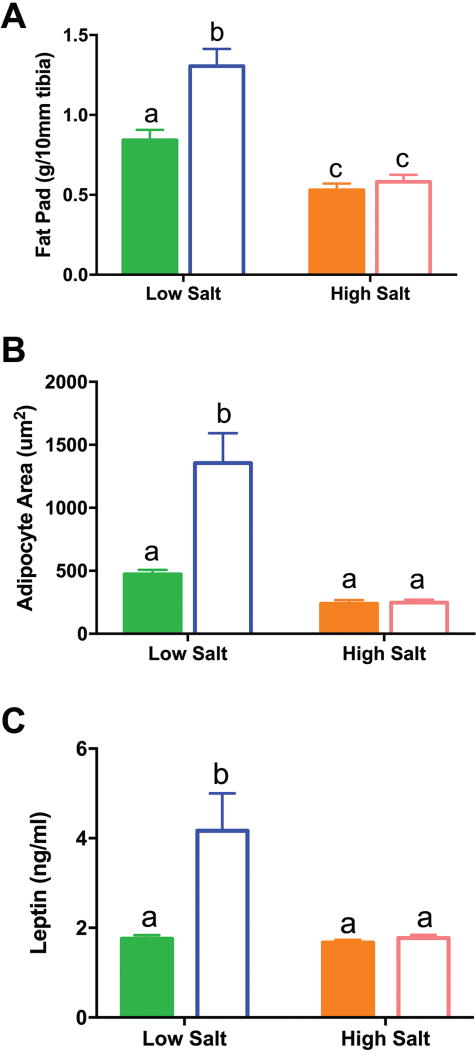
As tibia length did not differ among groups, body weight differences are likely due to alterations in adiposity. The combined retroperitoneal and perirenal fat pad weight was used as a marker of adiposity44, 45 and there was a significant interaction between salt and fat (F(1,101)=8.8, P<0.01). Consistent with body weight, rats on the HFLS diets had significantly more fat than those on the LFLS diet (55%; p< 0.0001; Figure 3) and fat pad weight correlated with body weight (r2=0.502; p<0.0001). In contrast, animals fed high salt had significantly lighter fat pads than LFLS (37%; p<0.01) or HFLS (55%; p<0.0001), indicating that high salt is inhibitory to fat formation and/or maintenance (Figure 3). The adiposity index (fat pad weight/100g body weight) was significantly lower in response to HS in both LF and HF groups (F(1,77)=5.22, p<0.0001). Similarly, when diets were alternated, the adiposity index was significantly lower in response to HS (Supplemental Figure).
Figure 3.
Changes in adiposity. (A) The fat pad in HFLS rats was significantly heavier than in both LFLS and HFHS animals. LFLS fat pads were also significantly heavier than fat pads from LFHS animals. (B): Adipocyte area was significantly higher in HFLS animals compared to LFLS and HFHS animals. (C): Plasma leptin concentrations were significantly higher in HFLS animals compared to LFLS and HFHS animals. Solid bars = Low Fat; Empty bars = High Fat. Differing letters indicate significant differences of at least p<0.05.
Adipocyte size also showed a significant interaction between salt and fat (F(1,13) = 8.98, P<0.01). Compared to LFLS, adipocytes were larger in HFLS (p<0.001), whereas adipocytes in HFHS were significantly smaller than HFLS (p<0.0001). While HFLS rats experienced hypertrophy of their adipocytes, the addition of high salt to high fat caused hypotrophy. There was a significant interaction between fat and salt (F(1,49)=7.78, p<0.01) on leptin. HFLS had significantly higher levels of leptin than LFLS (p<0.0001) and, commensurate with the adiposity index, had higher levels than HFHS (p<0.001). In keeping with the reduced adiposity in HFHS rats, their plasma leptin concentration was significantly (p<0.0001) reduced compared to HFLS rats. Corticosterone concentrations were not significantly different among diets (in ng/ml: LFLS: 36.2±8.4; LFHS: 36.1±12.6; HFLS: 22.7±9.7; HFHS: 30.7±11.0). Diet did not affect plasma osmolality (in mOsm/kg: LFLS: 279±1.3; LFHS: 280±6.7; HFLS: 277±3.4; HFHS: 282±2.4) or hematocrit (all diet means 51.2–52.1%).
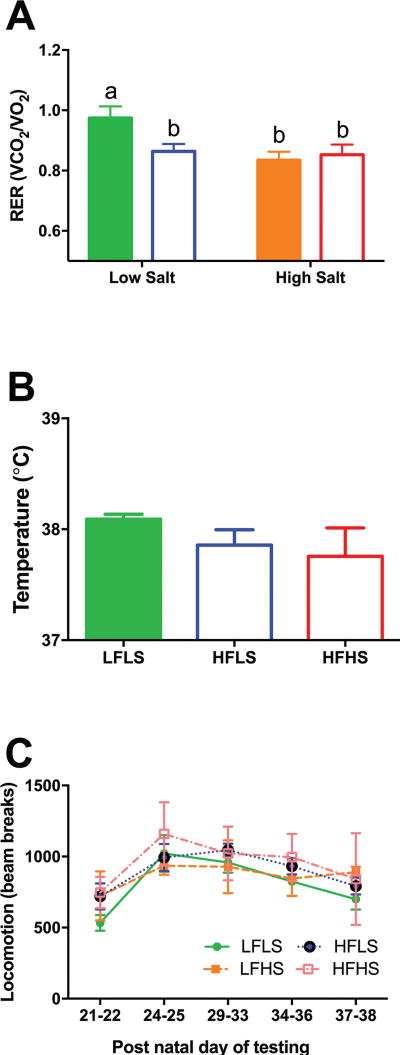
There was a significant effect of fat and salt on RER indicating a shift towards higher fat metabolism (F(1,23)=5.55, p<0.05). This effect was not cumulative as HFHS rats did not show an additional reduction in RER compared to LFHS and HFLS groups. However, there was no effect of diet on RMR, activity or body temperature (Figure 4).
Figure 4.
(A) Salt and/or fat reduced the respiratory exchange ratio. (B) Body temperature and (C) activity were not different among groups. Differing letters indicate significant differences of at least p<0.05.
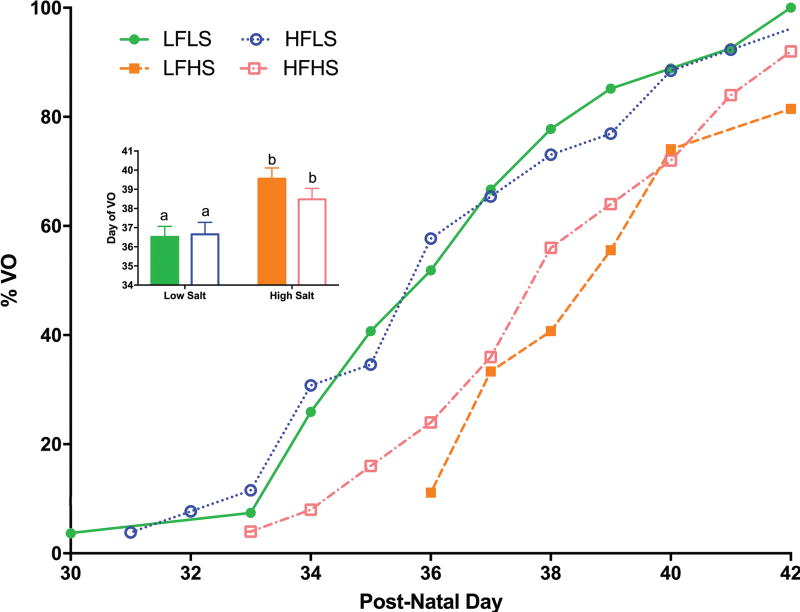
Salt significantly delayed puberty in both low and high fat groups (χ2=15.42, p<0.005; Figure 5). The timing of puberty in HFLS animals was not significantly different from LFLS. Salt still delayed puberty when diets were alternated with the control diet (data not shown).
Figure 5.
Salt significantly delayed vaginal opening (VO). Differing letters indicate significant differences of at least p<0.05.
Fat, without high salt, significantly increased heart weight (LFLS vs HFLS p<0.01; Table 1) and kidneys from LFHS were significantly larger than those from LS animals (p<0.001).
Table 1.
Organ Weights
| Diet | Heart | Liver | Spleen | Kidneys | Ov+Ut | Fat | Tibia (mm) | Length (mm) | |
|---|---|---|---|---|---|---|---|---|---|
| LFLS | g | 0.84 ± 0.07 | 7.91 ± 0.73 | 0.55 ± 0.05 | 1.80 ± 0.11 | 0.42 ± 0.03 | 2.74 ± 0.45 | 32.4 ± 0.6 | 183.4 ± 3.9 |
| g/10mm tibia | 0.26 ± 0.02 | 2.444 ± 0.22 | 0.17 ± 0.01 | 0.56 ± 0.03 | 0.13 ± 0.01 | 0.84 ± 0.13 | |||
| g/100m body length | 0.46 ± 0.03 | 4.31 ± 0.36 | 0.30 ± 0.02 | 0.99 ± 0.06 | 0.23 ± 0.02 | 1.48 ± 0.23 | |||
| LFHS | g | 0.90 ± 0.07 | 7.59 ± 0.5 | 0.49 ± 0.04 | 2.04 ± 0.14* | 0.37 ± 0.04 | 1.68 ± 0.28* | 31.6 ± 0.6 | 179.9 ± 3.0 |
| g/10mm tibia | 0.29 ± 0.02 | 2.40 ± 0.15 | 0.15 ± 0.01 | 0.65 ± 0.04* | 0.12 ± 0.01 | 0.53 ± 0.08* | |||
| g/100m body length | 0.50 ± 0.03 | 4.21 ± 0.28 | 0.27 ± 0.02 | 1.14 ± 0.08* | 0.21 ± 0.02 | 0.90 ± 0.14* | |||
| HFLS | g | 1.01 ± 0.13* | 8.70 ± 0.66† | 0.59 ± 0.07 † | 1.81 ± 0.11† | 0.44 ± 0.05 | 4.14 ± 0.7 *† | 31.8 ± 0.6 | 186.8 ± 3.8† |
| g/10mm tibia | 0.32 ± 0.04* | 2.74 ± 0.2† | 0.18 ± 0.02† | 0.57 ± 0.03† | 0.14 ± 0.02 | 1.31 ± 0.22*† | |||
| g/100m body length | 0.52 ± 0.05 | 4.71 ± 0.33 | 0.30 ± 0.03 | 0.98 ± 0.05† | 0.24 ± 0.03 | 2.18 ± 0.35*† | |||
| HFHS | g | 0.94 ± 0.09 | 7.69 ± 0.41 | 0.46 ± 0.03‡ | 1.92 ± 0.13 | 0.39 ± 0.04 | 1.85 ± 0.3*‡ | 31.7 ± 0.5 | 179.1 ± 3.5‡ |
| g/10mm tibia | 0.30 ± 0.03 | 2.43 ± 0.13 | 0.15 ± 0.01‡ | 0.61 ± 0.04 | 0.12 ± 0.01 | 0.58 ± 0.09*‡ | |||
| g/100m body length | 0.52 ± 0.04 | 4.30 ± 0.23 | 0.26 ± 0.02 | 1.07 ± 0.06 | 0.22 ± 0.02 | 1.03 ± 0.15*‡ |
significantly different from LFLS (p<0.05)
significantly different from LFHS (p<0.05)
significantly different from HFLS (p<0.05)
Discussion
Contrary to our initial hypothesis, salt did not increase weight gain in juvenile female rats. As expected, rats fed a high fat diet gained more weight than their low fat-fed counterparts but when salt was added to the high fat diet, the high fat-induced increase was lost. The effect of salt was not due to impaired growth as tibia length42 was unaffected by salt and there was no decrease in food intake or digestive efficiency. Rather, high salt decreased fat pad weight, at least in part, by decreasing adipocyte size; leptin concentrations reflected these changes in adiposity. It was also noteworthy that high salt significantly delayed puberty, regardless of the fat content of the diet. A simple unifying explanation for the salt-mediated anti-adiposity and delayed puberty outcomes would have been if the stress axis was activated but, as recently reported in male mice46, HS induced no differences in basal corticosterone concentrations.
Effect of a high fat diet
Most studies concur that a high fat diet increases body weight (for review see47). Our study shows that this effect is extremely rapid becoming evident within 10 days. Other studies, using the same percentage of high fat in the diet, report differences in rodent weights after 3 to 9 weeks48, 49. Female animals are more responsive to high fat diets and put on weight more rapidly than males47. Indirect measurements of adiposity, including BMI and the Lee index, did not differentiate groups and were unreliable in our study. Although a high fat diet has been reported to increase BMI50, this was not evident in our study even though the fat pad weight had increased by 60% with significant hypertrophy of the adipocytes. The inability of BMI to serve as a marker of significant adiposity changes during puberty has been reported previously in male rats51. Similarly, the Lee index has been reported to significantly correlate with adiposity43, but this was unaffected by the high fat diet in the present study. It is clearly apparent that the most reliable measure of adiposity in pubertal rats is body fat. Here, as in other studies52, a high fat diet caused larger fat pads due to, at least in part, adipocyte hypertrophy.
Leptin concentrations correlated with adiposity, which concurs with previous studies53. The reduced food intake due to higher calorie content is consistent with previous studies and this has been attributed to the negative feedback effects of leptin53, 54. If these animals were retained on the high fat diet for longer, food intake may have increased because of the shift towards leptin resistance53, 55. Rats on the high fat diet excreted more calories than low fat fed rats, which is consistent with increased fat consumption. In agreement with earlier studies56, 57, activity was unaffected by high fat feeding and body temperature was also unchanged.
High fat did not alter the timing of puberty, which does not agree with many34, 58, but not all59, 60, previous studies. This difference may be due to the control diet, as our rats went through puberty earlier than in previous studies. For example, Li et al34, using the same rats, reported that puberty onset occurred at pnd40. One speculation is that control diets are not comparable61. Thus, it is possible that our rats are already at the earliest puberty onset that is physiologically possible.
Effect of a high salt and low fat diet
Although salt did not affect weight gain in rats on low fat, there was evidence of a reduction after 2 weeks on the diet. This trend was reflected in the fat pad weights and adipocyte sizes, which were both significantly decreased by high salt. The effect of salt on body weight in rodents on control (low fat) diets is equivocal. Our study concurs with several previous investigations19, 46, 62, 63 showing no difference in body weight. However, others have reported a reduction37, 38, or an increase64, in body weight in response to high salt low fat diet. A recent study46 noted that that only when saline and not tap water is provided to male mice on a HS diet, does bodyweight decrease. We cannot discount the possibility that a lower salt content than we have used could increase adiposity. In this context, Dobrian et al15 reported that a 2% NaCl diet increased adipocyte cell size from an unspecified adipose depot. Additionally, a potential salt-mediated increase in adiposity could be transient. Fonseca et al16 described an increase in fat pad mass that was evident at 6 weeks but was no longer discernible after 9 weeks. As the aforementioned studies have used male rodents, and the current study has used female rats, the disparities noted may be due to sex differences on the effect of salt on adiposity.
It has been reported that high salt may increase food intake37. However, consistent with other studies62, 65, we found no effect of salt on food intake. It is noteworthy that food intake is elevated if the salt content of the drinking water is increased on top of a HS diet46. High salt also did not affect digestive efficiency in our study, which does not concur with a recent study on male mice in which high salt decreased digestive efficiency18. These opposing outcomes may be due to sex and/or species differences. Unfortunately other previous studies have not investigated this important variable.
This is the first study to show that high salt delays the onset of puberty in rats. The physiological mechanism(s) causing this delay is unknown, but the delay in puberty could be due to the reported changes in metabolism or changes in salt-responsive neurohormones or neurotransmitters66. Leptin was not significantly decreased in animals fed high salt in a low fat background, and it would thus be difficult to contend that the loss of leptin contributes to this delay. There are, however, two neurotransmitters that are known to modulate reproduction that are also affected by salt. Hyperosmolarity associated with high salt intake stimulates vasopressin secretion67. Vasopressin also has profound inhibitory effects on the reproductive axis68. Secondly, NKB is necessary for normal reproductive function, and its receptor, NK3, is activated in response to salt loading31. As animals may desensitize to NKB69, this could explain the delayed puberty but the alternating diet experiment would not support this conjecture. One or both of these neurotransmitters may be involved in this salt-mediated delay in puberty and warrant further studies.
Effect of a high salt and high fat diet
Salt attenuates body weight gain in animals fed a high fat diet. Although reduced muscle mass is not precluded46, the decrease in fat pad weight and adipocyte size suggest that adipocyte hypotrophy is a major underlying cause for the weight reduction in our study. This intriguing outcome concurs with two recent studies on male mice. Grobe and colleagues18 reported an inverse correlation between salt intake and weight gain in mice on high fat diets. Although DeClercq et al19 also reported diminished weight gain in mice on a high fat high salt diet compared to high fat alone, they noted an increase in epididymal fat pad weight as well as adipocyte size but a decrease in mesenteric adipocyte size. Prior to these studies, high salt was hypothesized to have an additive effect to the obese-inducing high fat diet12–14, 50. However, a preliminary report on male rats fed high fat and high salt (36%kcal and 4% NaCl) had less epididymal fat than high fat alone70. It is noteworthy that in Japan, where mean sodium intake is as high as 10.5g/day13, 71, the lowest levels of obesity worldwide have been reported72. Moreover, our study suggests that efforts to reduce sodium intake in Japan may inadvertently be increasing adiposity in that nation73.
Rats on high salt diets consumed more calories for each gram of fat deposited in the fat pad. Although Grobe et al18 also reported reduced body weight data in male mice on a HFHS diet, the cause of lower adiposity in that study was attributed to a renin-angiotensin mediated reduction in digestive efficiency. We found no evidence of a salt-mediated decrease in digestive efficiency in our study on juvenile female rats. This outcome was also evident in both the fecal lipid content and fecal steatocrit analyses: HFHS did not differ from HFLS fed rats. In contrast, our study showed that high fat reduced digestive efficiency, and this effect was not dependent on salt content. Neither activity nor body temperature was affected by salt and although both salt and fat reduced the respiratory exchange ratio (indicative of increased fat metabolism), no further reduction was evident when salt and fat were combined. Clearly, the physiological mechanism(s) responsible for the salt-mediated reduction in adiposity in the juvenile female rat in both low and high fat diets requires further investigation.
In agreement with the effects of salt in rats on the low fat diet, salt also delayed puberty in rats fed the high fat diet. Thus, regardless of the presence of fat, high salt is inhibitory to puberty. It is interesting to speculate that potential differences in puberty onset in previous studies may be mediated, at least in part, by dietary salt content.
Conclusion
Our study shows that a high salt diet has an inhibitory action on adiposity that is most evident in high fat dietary conditions. In addition, our study provides compelling evidence for the first time that high salt delays puberty. Whether the underlying mechanism(s) mediating these effects on adiposity and reproduction is shared between these physiological systems is unknown but warrants further research. Clearly, if the physiological mechanisms responsible for the absence of adiposity in the presence of a high fat diet were understood, new avenues of intervention may be possible to combat the ever-growing obesity epidemic.
Supplementary Material
Supplementary Figure. (A) When experimental diets were alternated with the control LFLS diet, there was no significant change in weight relative to starting weight. (B) Calorie intake was significantly higher on the first 2 intakes of HF for the HFLS animals, after which there was no difference in calorie intake among groups. Salt reduced calorie intake in LFHS animals as they consumed more calories when given the control diet but this was not evident when rats were fed the HFHS diet. (C) Although HFLS had no effect on fat pad weight, this weight was reduced when rats were fed high salt (LFLS vs LFHS and HFLS vs HFHS both P<0.05)
Acknowledgments
Research was supported by National Institutes of Health grants P30 GM103398 and NS57823 awarded to F.W.F. An Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant #2P20GM103432 provided student support for M.R., M.S., R.S., and T.F.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to report.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults During 1980–2013: a Systematic Analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Flegal KM. Epidemiologic Trends in Overweight and Obesity. Endocrinology Metabolism Clinics of North America. 2003;32(4):741–60. doi: 10.1016/s0889-8529(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity Among Adults: United States, 2011–2012. NCHS Data Brief. 2013;(131):1–8. [PubMed] [Google Scholar]
- 4.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting Adult Obesity from Childhood Obesity: a Systematic Review and Meta-Analysis. Obesity Reviews. 2016;17(2):95–107. doi: 10.1111/obr.12334. [DOI] [PubMed] [Google Scholar]
- 5.Dunger DB, Ahmed ML, Ong KK. Effects of Obesity on Growth and Puberty. Best Practice & Research Clinical Endocrinology & Metabolism. 2005;19(3):375–90. doi: 10.1016/j.beem.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of Clinical Oncology Position Statement on Obesity and Cancer. Journal of Clinical Oncology. 2014;32(31):3568–3574. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss An Update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease From the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 8.Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR. Energy Balance and its Components: Implications for Body Weight Regulation. Am J Clin Nutr. 2012;95(4):989–994. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenvinkel P. Obesity a Disease with many Aetiologies Disguised in the Same Oversized Phenotype: has the Overeating Theory Failed? Nephrology Dialysis Transplantation. 2015;30(10):1656–1664. doi: 10.1093/ndt/gfu338. [DOI] [PubMed] [Google Scholar]
- 10.Comuzzie AG, Allison DB. The Search for Human Obesity Genes. Science. 1998;280(5368):1374–1377. doi: 10.1126/science.280.5368.1374. [DOI] [PubMed] [Google Scholar]
- 11.Popkin BM, Adair LS, Ng SW. Global Nutrition Transition and the Pandemic of Obesity in Developing Countries. Nutrition Reviews. 2012;70(1):3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song HJ, Cho YG, Lee H-J. Dietary Sodium Intake and Prevalence of Overweight in Adults. Metabolism. 2013;62(5):703–708. doi: 10.1016/j.metabol.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Zhu H, Pollock NK, Kotak I, Gutin B, Wang X, Bhagatwala J, et al. Dietary Sodium, Adiposity, and Inflammation in Healthy Adolescents. Pediatrics. 2014;133(3):e635–e642. doi: 10.1542/peds.2013-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann IS, Cubeddu LX. Salt and the Metabolic Syndrome. Nutrition, Metabolism and Cardiovascular Diseases. 2009;19(2):123–128. doi: 10.1016/j.numecd.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Dobrian AD, Schriver SD, Lynch T, Prewitt RL. Effect of Salt on Hypertension and Oxidative Stress in a Rat Model of Diet-Induced Obesity. American Journal Physiology Renal Physiology. 2003;285(4):F619–28. doi: 10.1152/ajprenal.00388.2002. [DOI] [PubMed] [Google Scholar]
- 16.Fonseca-Alaniz MH, Brito LC, Borges-Silva CN, Takada J, Andreotti S, Lima FB. High Dietary Sodium Intake Increases White Adipose Tissue Mass and Plasma Leptin in Rats. Obesity. 2007;15(9):2200–2208. doi: 10.1038/oby.2007.261. [DOI] [PubMed] [Google Scholar]
- 17.Fonseca-Alaniz MH, Takada J, Andreotti S, de Campos TBF, Campaña AB, Borges-Silva CN, et al. High Sodium Intake Enhances Insulin-stimulated Glucose Uptake in Rat Epididymal Adipose Tissue. Obesity. 2008;16(6):1186–1192. doi: 10.1038/oby.2008.69. [DOI] [PubMed] [Google Scholar]
- 18.Weidemann BJ, Voong S, Morales-Santiago FI, Kahn MZ, Ni J, Littlejohn NK, et al. Dietary Sodium Suppresses Digestive Efficiency via the Renin-Angiotensin System. Scientific Reports. 2015;5 doi: 10.1038/srep11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeClercq VC, Goldsby JS, McMurray DN, Chapkin RS. Distinct Adipose Depots from Mice Differentially Respond to a High-Fat, High-Salt Diet. Journal of Nutrition. 2016;146(6):1189–1196. doi: 10.3945/jn.115.227496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker MS, Li G, Kohorst JJ, Waterland RA. Fetal Growth Restriction Promotes Physical Inactivity and Obesity in Female Mice. International Journal of Obesity. 2015;39:98–104. doi: 10.1038/ijo.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplowitz PB. Link Between Body Fat and the Timing of Puberty. Pediatrics. 2008;121:S208–S217. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- 22.Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier Onset of Puberty in Girls: Relation to Increased Body Mass Index and Race. Pediatrics. 2001;108(2):347–53. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- 23.Biro FM, Greenspan LC, Galvez MP. Puberty in Girls of the 21st Century. Journal of Pediatric & Adolescent Gynecology. 2012;25(5):289–294. doi: 10.1016/j.jpag.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biro FM, Greenspan LC, Galvez MP, Pinney SM, Teitelbaum S, Windham GC, et al. Onset of Breast Development in a Longitudinal Cohort. Pediatrics. 2013;132(6):1019–1027. doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aksglaede L, Sorensen K, Petersen JH, Skakkebaek NE, Juul A. Recent Decline in Age at Breast Development: The Copenhagen Puberty Study. Pediatrics. 2009;123(5):e932–e939. doi: 10.1542/peds.2008-2491. [DOI] [PubMed] [Google Scholar]
- 26.Deardorff J, Gonzales NA, Christopher FS, Roosa MW, Millsap RE. Early Puberty and Adolescent Pregnancy: the Influence of Alcohol Use. Pediatrics. 2005;116(6):1451–6. doi: 10.1542/peds.2005-0542. [DOI] [PubMed] [Google Scholar]
- 27.Ge X, Conger RD, Elder GH. Coming of Age Too Early: Pubertal Influences on Girls' Vulnerability to Psychological Distress. Child Development. 1996;67(6):3386–3400. [PubMed] [Google Scholar]
- 28.Williams RM, Ong KK, Dunger DB. Polycystic Ovarian Syndrome During Puberty and Adolescence. Moleculr Cell Endocrinology. 2013;373(1–2):61–67. doi: 10.1016/j.mce.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI. Growth patterns and the risk of breast cancer in women. New England Journal of Medicine. 2004;351(16):1619–26. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 30.Herman-Giddens ME. The Enigmatic Pursuit of Puberty in Girls. Pediatrics. 2013;132(6):1125–1126. doi: 10.1542/peds.2013-3058. [DOI] [PubMed] [Google Scholar]
- 31.Haley GE, Flynn FW. Tachykinin NK3 Receptor Contribution to Systemic Release of Vasopressin and Oxytocin in Response to Osmotic and Hypotensive Challenge. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2007;293(2):R931–7. doi: 10.1152/ajpregu.00196.2007. [DOI] [PubMed] [Google Scholar]
- 32.Grachev P, Millar RP, O'Byrne KT. The Role of Neurokinin B Signalling in Reproductive Neuroendocrinology. Neuroendocrinology. 2014;99(1):7–17. doi: 10.1159/000357734. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy GC, Mitra J. Body Weight and Food Intake as Initiating Factors for Puberty in the Rat. Journal of Physiology. 1963;166(2):408–418. doi: 10.1113/jphysiol.1963.sp007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XF, Lin YS, Kinsey-Jones JS, O'Byrne KT. High-Fat Diet Increases LH Pulse Frequency and Kisspeptin-Neurokinin B Expression in Puberty-Advanced Female Rats. Endocrinology. 2012;153(9):4422–31. doi: 10.1210/en.2012-1223. [DOI] [PubMed] [Google Scholar]
- 35.Porter JP, King SH, Honeycutt AD. Prenatal High-Salt Diet in the Sprague-Dawley Rat Programs Blood Pressure and Heart Rate Hyperresponsiveness to Stress in Adult Female Offspring. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2007;293(1):R334–R342. doi: 10.1152/ajpregu.00887.2006. [DOI] [PubMed] [Google Scholar]
- 36.Makita K, Takahashi K, Karara A, Jacobson HR, Falck JR, Capdevila JH. Experimental and/or Genetically Controlled Alterations of the Renal Microsomal Cytochrome P450 Epoxygenase Induce Hypertension in Rats Fed a High Salt Diet. Journal of Clinical Investigation. 1994;94(6):2414–2420. doi: 10.1172/JCI117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coêlho MS, Passadore MD, Gasparetti AL, Bibancos T, Prada PO, Furukawa LL, et al. High- or Low-Salt Diet from Weaning to Adulthood: Effect on Body Weight, Food Intake and Energy Balance in Rats. Nutrition Metabolism Cardiovascular Disease. 2006;16(2):148–155. doi: 10.1016/j.numecd.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Lenda DM, Sauls BA, Boegehold MA. Reactive Oxygen Species may Contribute to Reduced Endothelium-Dependent Dilation in Rats Fed High Salt. American Journal of Physiology - Heart and Circulatory Physiology. 2000;279(1):H7–H14. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- 39.Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative Stress in a Rat Model of Obesity-Induced Hypertension. Hypertension. 2001;37(2):554–560. doi: 10.1161/01.hyp.37.2.554. [DOI] [PubMed] [Google Scholar]
- 40.Lusk G. The Elements of the Science of Nutrition. 4. W.B. Saunders; 1928. [Google Scholar]
- 41.Burnett CML, Grobe JL. Dietary effects on resting metabolic rate in C57BL/6 mice are differentially detected by indirect (O2/CO2 respirometry) and direct calorimetry. Mol Metab. 2014;3(4):460–464. doi: 10.1016/j.molmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of Tibial Length to Quantify Cardiac Hypertrophy: Application in the Aging Rat. American Journal of Physiology - Heart and Circulatory Physiology. 1982;243(6):H941–H947. doi: 10.1152/ajpheart.1982.243.6.H941. [DOI] [PubMed] [Google Scholar]
- 43.Bernardis LL. Prediction of Carcass Fat, Water and Lean Body Mass from Lee's 'Nutritive Ratio' in Rats with Hypothalamic Obesity. Experientia. 1970;26(7):789–790. doi: 10.1007/BF02232553. [DOI] [PubMed] [Google Scholar]
- 44.Hausman DB, Fine JB, Tagra K, Fleming SS, Martin RJ, DiGirolamo M. Regional Fat Pad Growth and Cellularity in Obese Zucker Rats: Modulation by Caloric Restriction. Obesity Research. 2003;11(5):674–682. doi: 10.1038/oby.2003.96. [DOI] [PubMed] [Google Scholar]
- 45.Hung C-S, Lee J-K, Yang C-Y, Hsieh H-R, Ma W-Y, Lin M-S, et al. Measurement of Visceral Fat: Should We Include Retroperitoneal Fat? PLoS ONE. 2014;9(11):e112355. doi: 10.1371/journal.pone.0112355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitada K, Daub S, Zhang Y, Klein JD, Nakano D, Pedchenko T, et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J Clin Invest. 2017 doi: 10.1172/JCI88532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hariri N, Thibault L. High-Fat Diet-Induced Obesity in Animal Models. Nutr Res Rev. 2010;23(2):270–99. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 48.Boyle CN, Rossier MlM, Lutz TA. Influence of High-Fat Feeding, Diet-Induced Obesity, and Hyperamylinemia on the Sensitivity to Acute Amylin. Physiology and Behavior. 2011;104(1):20–28. doi: 10.1016/j.physbeh.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-Related Insulin Resistance. Journal of Clinical Investigation. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bao P, Liu G, Wei Y. Association between IL-6 and Related Risk Factors of Metabolic Syndrome and Cardiovascular Disease in Young Rats. International Journal of Clinial Experimental Medicine. 2015;8(8):13491–13499. [PMC free article] [PubMed] [Google Scholar]
- 51.Engelbregt MJ, van Weissenbruch MM, Popp-Snijders C, Lips P, Delemarre-van de Waal HA. Body Mass Index, Body Composition, and Leptin at Onset of Puberty in Male and Female Rats after Intrauterine Growth Retardation and After Early Postnatal Food Restriction. Pediatric Research. 2001;50(4):474–8. doi: 10.1203/00006450-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, et al. PPARγ Mediates High-Fat Diet-Induced Adipocyte Hypertrophy and Insulin Resistance. Molecular Cell. 1999;4(4):597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 53.Lin S, Thomas TC, Storlien LH, Huang XF. Development of High Fat Diet-Induced Obesity and Leptin Resistance in C57Bl/6J Mice. International Journal of Obesity. 2000;24(5) doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 54.Assaad H, Yao K, Tekwe CD, Feng S, Bazer FW, Zhou L, et al. Analysis of Energy Expenditure in Diet-Induced Obese Rats. Frontiers in Bioscience. 2014;19:967–985. doi: 10.2741/4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Widdowson PS, Upton R, Buckingham R, Arch J, Williams G. Inhibition of Food Response to Intracerebroventricular Injection of Leptin Is Attenuated in Rats With Diet-Induced Obesity. Diabetes. 1997;46(11):1782–1785. doi: 10.2337/diab.46.11.1782. [DOI] [PubMed] [Google Scholar]
- 56.McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA, Flatt PR. GIP Receptor Antagonism Reverses Obesity, Insulin Resistance, and Associated Metabolic Disturbances Induced in Mice by Prolonged Consumption of High-Fat Diet. American Journal of Physiology - Endocrinology and Metabolism. 2007;293(6):E1746–E1755. doi: 10.1152/ajpendo.00460.2007. [DOI] [PubMed] [Google Scholar]
- 57.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, et al. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metabolism. 2007;6(5):414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Frisch RE, Hegsted DM, Yoshinaga K. Body Weight and Food Intake at Early Estrus of Rats on a High-Fat Diet. Proceedings of the National Academy of Sciences. 1975;72(10):4172–6. doi: 10.1073/pnas.72.10.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramaley JA. Puberty Onset in Males and Females Fed a High Fat Diet. Proceedings of the Society for Experimental Biology and Medicine. 1981;166(2):294–296. doi: 10.3181/00379727-166-41062. [DOI] [PubMed] [Google Scholar]
- 60.Lie MEK, Overgaard A, Mikkelsen JD. Effect of a Postnatal High-Fat Diet Exposure on puberty Onset, Estrous Cycle Regularity, and Kisspeptin Expression in Female Rats. Reproductive Biology. 2013;13(4):298–308. doi: 10.1016/j.repbio.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Feng Li X, Lin YS, Kinsey-Jones JS, O'Byrne KT. High-Fat Diet Increases LH Pulse Frequency and Kisspeptin-Neurokinin B Expression in Puberty-Advanced Female Rats. Endocrinology. 153(9):4422–4431. doi: 10.1210/en.2012-1223. [DOI] [PubMed] [Google Scholar]
- 62.Ogihara T, Asano T, Ando K, Chiba Y, Sekine N, Sakoda H, et al. Insulin Resistance With Enhanced Insulin Signaling in High-Salt Diet Fed Rats. Diabetes. 2001;50(3):573–583. doi: 10.2337/diabetes.50.3.573. [DOI] [PubMed] [Google Scholar]
- 63.Nurkiewicz TR, Boegehold MA. High Salt Intake Reduces Endothelium-Dependent Dilation of Mouse Arterioles Via Superoxide Anion Generated from Nitric Oxide Synthase. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2007;292(4):R1550–R1556. doi: 10.1152/ajpregu.00703.2006. [DOI] [PubMed] [Google Scholar]
- 64.Lasheen NN. Pancreatic Functions in High Salt Fed Female Rats. Physiological Reports. 2015;3(7):e12443. doi: 10.14814/phy2.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding Y, Lv J, Mao C, Zhang H, Wang A, Zhu L, et al. High-Salt Diet during Pregnancy and Angiotensin-Related Cardiac Changes. Journal of Hypertension. 2010;28(6):1290–1297. doi: 10.1097/HJH.0b013e328337da8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pitynski D, Flynn FW, Skinner DC. Does Salt have a Permissive Role in the Induction of Puberty? Med Hypotheses. 2015;85(4):463–467. doi: 10.1016/j.mehy.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greenwood MP, Mecawi AS, Hoe SZ, Mustafa MR, Johnson KR, Al-Mahmoud GA, et al. A Comparison of Physiological and Transcriptome Responses to Water Deprivation and Salt Loading in the Rat Supraoptic Nucleus. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2015;308(7):R559–R568. doi: 10.1152/ajpregu.00444.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cates Forsling, O’Byrne Stress-Induced Suppression of Pulsatile Luteinising Hormone Release in the Female Rat: Role of Vasopressin. Journal of Neuroendocrinology. 1999;11(9):677–683. doi: 10.1046/j.1365-2826.1999.00380.x. [DOI] [PubMed] [Google Scholar]
- 69.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B Stimulates GnRH Release in the Male Monkey (Macaca mulatta) and Is Colocalized with Kisspeptin in the Arcuate Nucleus. Endocrinology. 151(9):4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin C, MacDonell R, Speed J, Pollock D. Synergy of High Salt and High Fat Diet on Kidney Injury and Adiposity. The FASEB Journal. 2014;28(1 Supplement):1086–1. [Google Scholar]
- 71.Okuda M, Asakura K, Sasaki S, Shinozaki K. Twenty-Four-Hour Urinary Sodium and Potassium Excretion and Associated Factors in Japanese Secondary School Students. Hypertension Research. 2016;39(7):524–529. doi: 10.1038/hr.2016.24. [DOI] [PubMed] [Google Scholar]
- 72.Yoshiike N, Miyoshi M. Epidemiological Aspects of Overweight and Obesity in Japan--International Comparisons. Japanese Journal of Clinical Medicine. 2013;71(2):207–216. [PubMed] [Google Scholar]
- 73.Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and Classification of Obesity in Japan and Asia-Oceania. Asia Pacific Journal of Clinical Nutrition. 2002;11:S732–S737. doi: 10.1159/000088200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. (A) When experimental diets were alternated with the control LFLS diet, there was no significant change in weight relative to starting weight. (B) Calorie intake was significantly higher on the first 2 intakes of HF for the HFLS animals, after which there was no difference in calorie intake among groups. Salt reduced calorie intake in LFHS animals as they consumed more calories when given the control diet but this was not evident when rats were fed the HFHS diet. (C) Although HFLS had no effect on fat pad weight, this weight was reduced when rats were fed high salt (LFLS vs LFHS and HFLS vs HFHS both P<0.05)