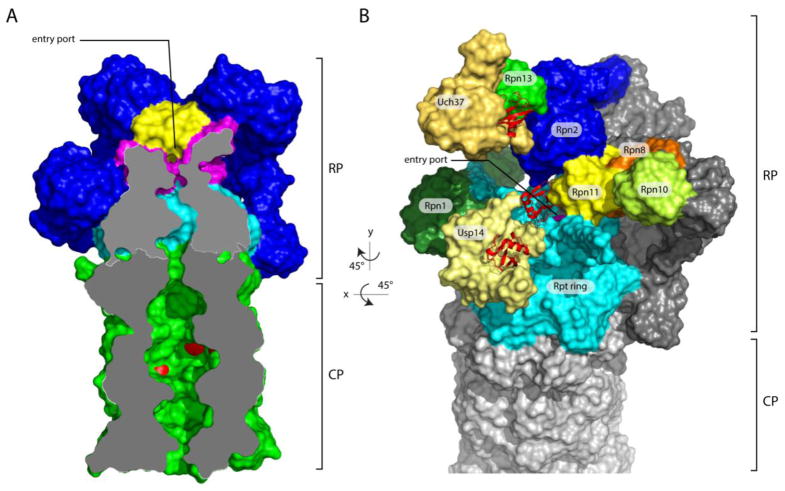
Figure 1. Overview of the axial channel and the position of the three proteasomal-associated DUBs on the human proteasome.
(A) The axial channels of the RP and CP of the human proteasome in a substrate-translocating, s3-like state. This representation was generated using structural data from PDB: 5T0J [26]. The grey surface represents a medial cut-away plane, which is only applied to the CP and the Rpt ring. The substrate entry port is formed by the OB domains, also known as N-domains. Rpn11 is painted yellow, the OB domains of the Rpt ring are in magenta, the ATPase domains are in cyan, and the CP is in green. The proteolytic active sites β1, β2 and β5’ are highlighted in red. To allow visualization of the internal space of the proteasome, certain subunits were removed. (B) Representation of the structure of the human s1 proteasome as determined by Huang et al. (PDB: 5GJQ) [54]. The three DUBs are shown in shades of yellow, whereas the three Ub/Ubl receptors are colored in various shades of green. Ubiquitin bound to the DUBs is shown in red ribbon representations. Rpn8, part of the MPN heterodimer with Rpn11, is shown in orange. Rpn13, Uch37, and ubiquitin were modeled onto the RP based on the position of Rpn13 on the yeast proteasome [53] (PDB: 5A5B) and the co-crystal structure of Uch37-Rpn13 (PDB: 4UEL, PDB: 4WLQ) [60,61]. The substrate entry port was built digitally by filling the hole with H2O molecules using HOLLOW [157].