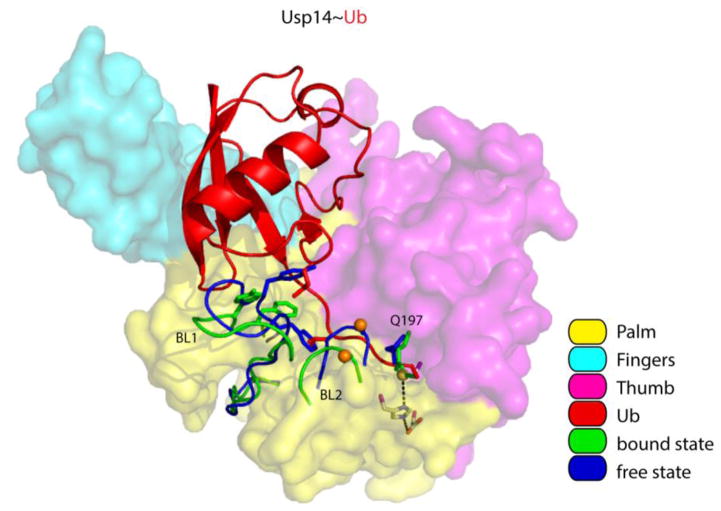
Figure 4. Displacement of the BL1 and BL2 loops activates Usp14.
Overview of Usp14 and UbAl, based on PDB: 2AYN and PDB: 2AYO [70]. The surfaces of the Palm, Thumb and Fingers domains were made semi-transparent to show the side chains of the catalytic triad. Usp14 regions that reorient upon binding (including the BL1 and BL2 loop) are displayed in blue for the free state and in green for the bound state. We note that the side chain of Q197 in the Thumb domain of Usp14 flips when Usp14 binds ubiquitin. The thiol group of the catalytic cysteine is shown as a yellow sphere, and phosphorylation site Ser432 on BL2 as an orange sphere. The isopeptide bond between UbAl and the catalytic cysteine is in green. Several side chains on the BL1 loop that interfere with ubiquitin binding are shown as stick models in addition to the cartoon backbone in both blue (free) and green (bound). Other color coding is explained in the key.