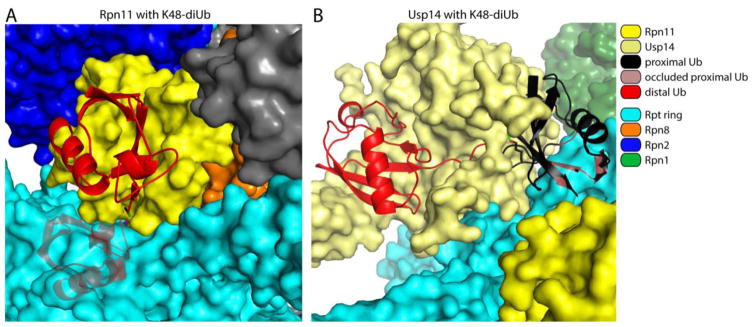
Figure 6. Proteasome-associated Rpn11 and Usp14 cannot accommodate folded proximal ubiquitin molecules.
Molecular modeling was used to show potential steric exclusion for the proximal ubiquitin within a di-ubiquitin element docked at either Rpn11 (A) or Usp14 (B, modified from [35]) within the proteasome. In short, isopeptide from di-ubiquitin (PDB: 1TBE [158]) was fit to the C-terminus of the bound distal ubiquitin on Rpn11 (PDB: 5U4P [34]) and Usp14 (PDB: 5GJQ [54]). The position of the proximal ubiquitin was then varied through all possible configurations of the Lys side chain for all the lysines in ubiquitin, from which no clash-free position could be found. The configurations for the proximal ubiquitins with the least steric conflict are shown, using a K48-linked ubiquitin-dimer as an example. The proximal ubiquitin is painted black while the portion that clashes with the Rpt subunits is shaded grey with red highlights. The isopeptide bond between the two ubiquitin molecules is in green.