Figure 2. Schematic of ubiquitin modifying enzymes and ubiquitination events regulating NLRP3 inflammasome activity.
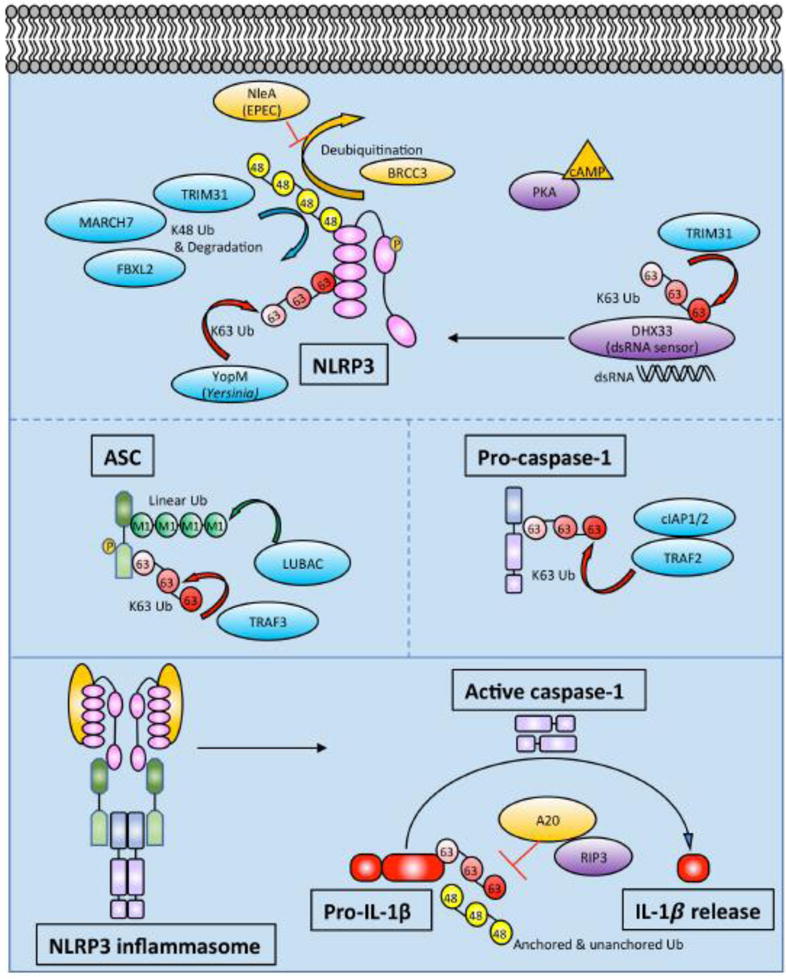
In addition to upregulating NLRP3 and IL1β mRNA, priming also leads to NLRP3 deubiquitination. NLRP3 is modified by K48- and K63-linked Ub as described in the text. Phosphorylation of NLRP3 may be linked to its ubiquitination status. Prior to NLRP3 inflammasome assembly, ASC gets phosphorylated and linearly ubiquitinated. ASC can also undergo K63-linked ubiquitination by TRAF3. Pro-caspase-1 undergoes K63-linked polyubiquitination by cIAP1, cIAP2, and TRAF2. After NLRP3 inflammasome assembly, active caspase-1 cleaves pro-IL-1β to IL-1β. Pro-IL-1β undergoes K63-linked polyubiquitination and binds unanchored Ub chains. A20 restricts polyubiquitination of pro-IL-1β in a RIPK3-dependent manner. The exact character and composition of heterogeneous ubiquitin chains in the inflammasome are not well understood.
