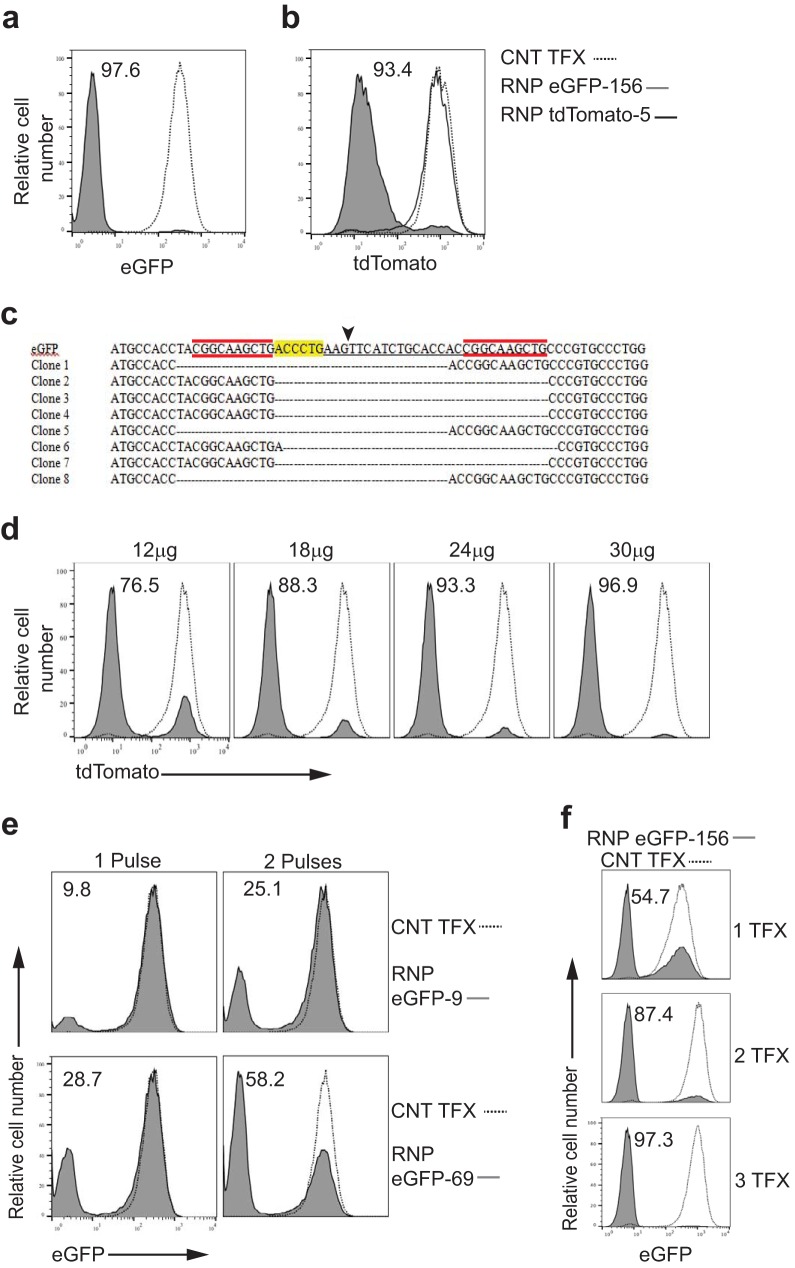
FIG 1 .
SaCas9/sgRNA delivery and gene knockout in T. cruzi epimastigotes. (a) Epimastigotes from T. cruzi expressing eGFP were electroporated with water (control transfection [CNT TFX]; dotted line) or RNP complexes containing SaCas9 and an sgRNA designated eGFP-156, targeting eGFP (RNP eGFP-156; gray filled) (see Table S1 in the supplemental material for description of all sgRNAs). (b) Epimastigotes from T. cruzi expressing tdTomato were electroporated with water (dashed line) or RNP complexes containing SaCas9 and sgRNA eGFP-156 (gray line) or an sgRNA designated tdTomato-5, targeting tdTomato (RNP tdTomato-5; grey filled). (c) DNA sequences of clonal lines from flow-sorted eGFP-negative parasites from the experiment whose results are shown in panel a. Arrowhead identifies the site of sgRNA eGFP-156-directed SaCas9 cleavage, highlighting identifies the PAM sequence, and red identifies microhomology regions. (d) Epimastigotes from T. cruzi expressing tdTomato were electroporated with increasing amounts of RNP complexes (SaCas9 and sgRNA tdTomato-5). The quantity of recombinant SaCas9 for each transfection is indicated (molar ratio of SaCas9/sgRNA is 1:1 in all cases). (e) Epimastigotes from T. cruzi expressing eGFP were electroporated with water (dashed line) or RNP complexes containing SaCas9 and sgRNA eGFP-9 or eGFP-69, targeting eGFP (gray filled). Parasites received 1 or 2 pulses of electroporation (the 2 pulses were applied consecutively). (f) Epimastigotes from T. cruzi expressing eGFP were electroporated with water (dashed line) or RNP complexes containing SaCas9 and an sgRNA targeting eGFP (eGFP-156; gray filled). Parasites received 1, 2, or 3 electroporations with a 2-day interval between each. Loss of eGFP or tdTomato fluorescence was determined 7 days after the first transfection. Each experiment was replicated 1 to 3 times with similar results.