FIG 2 .
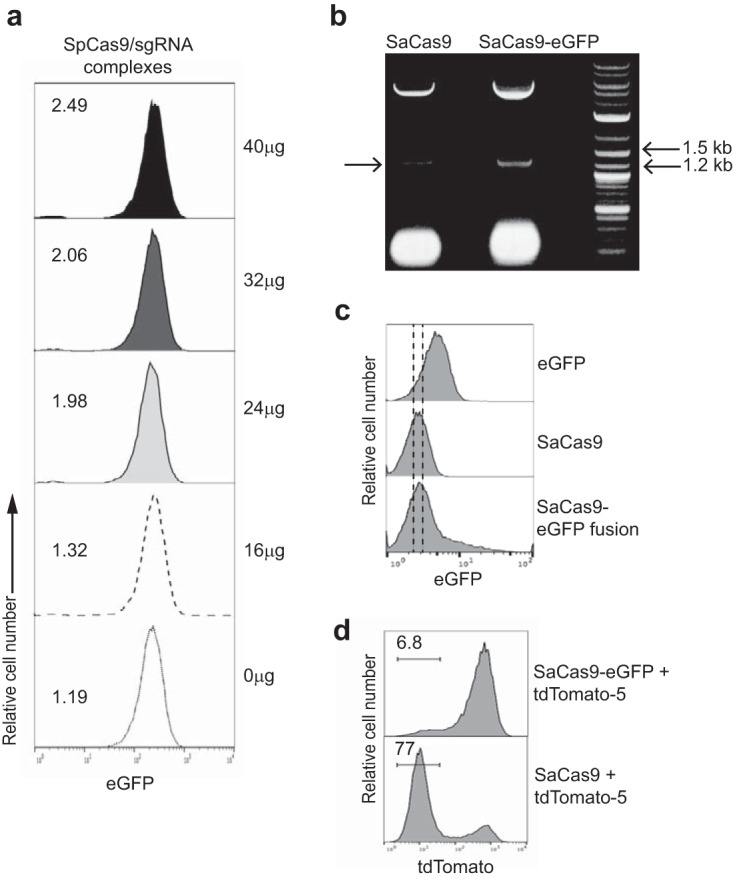
Cas9-RNP size impacts knockout efficiency. (a) Epimastigotes from T. cruzi expressing eGFP were electroporated with water (0 µg) or RNP complexes containing SpCas9 and sgRNA eGFP-153, targeting eGFP (sgRNA designed for association with SpCas9) in increasing amounts (as indicated, always keeping the molar ratio of SpCas9/sgRNA at 1:1). (b) Image of agarose gel showing cleavage product from in vitro nuclease activity assay performed with SaCas9 and SaCas9-eGFP fusion protein. Arrow on left indicates cleavage product. DNA markers are in the right lane. This experiment was performed twice, using the same batch of SaCas-eGFP fusion protein and different batches of SaCas9 protein. (c) Wild-type epimastigotes of T. cruzi were electroporated with eGFP, SaCas9, or SaCas9-eGFP fusion proteins. eGFP fluorescence was monitored immediately after electroporation by flow cytometry. A right shift in fluorescence of the entire parasite population in the eGFP-transfected group but not in those transfected with SaCas9-eGFP indicates the limited entry of SaCas9-eGFP into T. cruzi under these conditions. The experiment was performed 2 times with similar results. (d) Epimastigotes of T. cruzi expressing tdTomato were electroporated with RNP complexes containing SaCas9-eGFP or SaCas9 and sgRNA tdTomato-5 targeting tdTomato. Loss of tdTomato fluorescence was assessed 7 days posttransfection by flow cytometry. Loss of fluorescence was only observed in the parasites transfected with SaCas9 (approximately 124-kDa protein) and not in the parasites transfected with SaCas9-eGFP (153-kDa protein). The experiment was performed 2 times with similar results.
