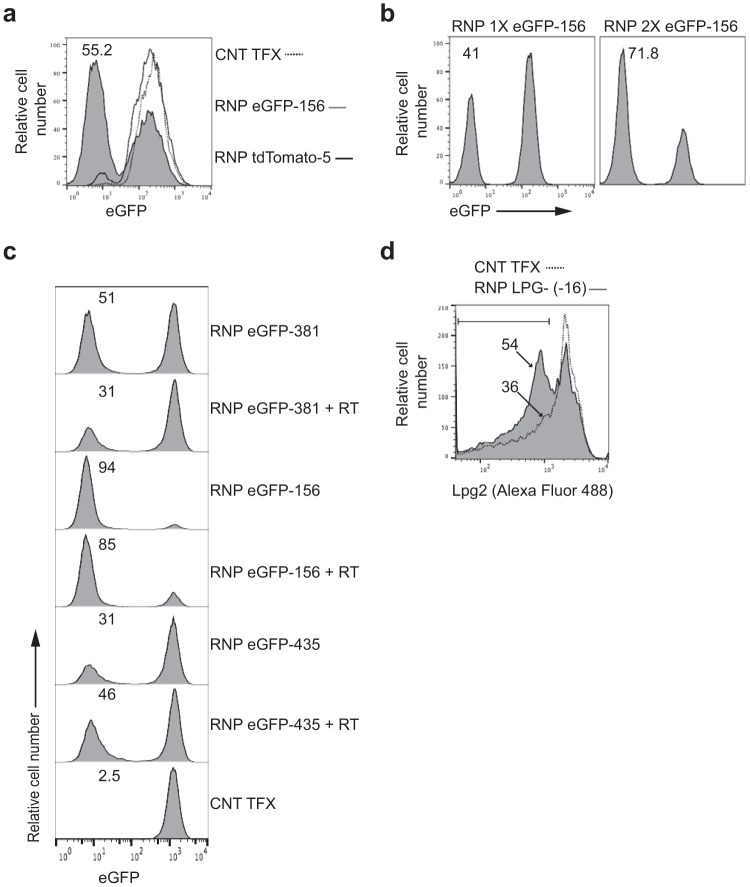
FIG 6 .
SaCas9/sgRNA delivery and gene knockout in the kinetoplastids Trypanosoma brucei and Leishmania major. (a) eGFP-expressing bloodstream forms of T. brucei were electroporated with one pulse in the presence of water (dashed line) or of RNP complexes containing SaCas9 and sgRNA eGFP-156, targeting eGFP (gray filled), or sgRNA tdTomato-5, targeting tdTomato (solid blackline). Loss of eGFP fluorescence was determined 5 days posttransfection by flow cytometry. (b) Five days after the first electroporation with SaCas9 and sgRNA eGFP-156, targeting eGFP, the mixed population of parasites was electroporated a second time with water (RNP 1× eGFP-156) or with the RNP complex targeting eGFP (RNP 2× eGFP-156). Loss of eGFP fluorescence was determined 5 days after the second electroporation by flow cytometry. The experiments whose results are shown in panels a and b were each performed once. (c) eGFP-positive L. major log-phase promastigotes were electroporated with RNP complexes containing SaCas9 and gRNAs targeting the eGFP gene at 3 positions (381, 156, and 435 bp downstream from the GFP start codon), with and without corresponding repair templates that insert a stretch of 3 in-frame stop codons at the gRNA target sites. Loss of eGFP fluorescence was determined 5 days posttransfection by flow cytometry. (d) L. major promastigotes were electroporated with RNP complexes containing SaCas9 and a gRNA targeting the lpg2 gene, plus a corresponding repair template that inserts a stretch of 3 in-frame stop codons at the gRNA target site. Loss of parasite surface lipophosphoglycan was assessed at day 10 posttransfection by staining with WIC79.3 monoclonal antibody and Alexa Fluor 488 IgG secondary antibody. The experiments whose results are shown in panels c and d were replicated 2 and 4 times, respectively.