Figure 1.
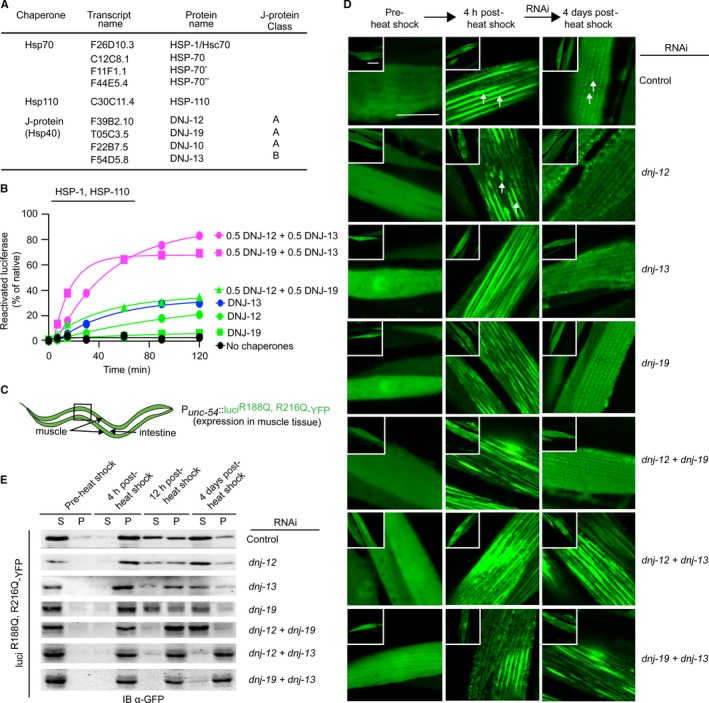
In vitro and in vivo J‐protein class cooperation. (A) Table summarizing cytosolic and nuclear molecular chaperones from Caenorhabditis elegans used in the study. (B) In vitro reactivation of preformed luciferase aggregates by HSP‐1, HSP‐110, and the indicated J‐protein(s). Control (absence of chaperones) is depicted in black. Single class A and class B J‐protein containing reactions are depicted in green and blue, respectively. Representative experiment shown; N = 3. (C) Cartoon depicting the targeted expression of aggregation‐sensor luciR188Q,R216Q‐YFP in C. elegans. (D) Fluorescent images of C. elegans muscle cells expressing luciR188Q,R216Q‐YFP. Single and double RNAi knockdowns (KD) of cytosolic canonical class A and class B J‐proteins indicated in text on right. Left, middle, and right image columns show solubility of luciferase prior to heat shock, 4 h post‐heat shock, and 4 days post‐heat shock. White arrows show luciferase aggregates. Control animals fed with empty RNAi vector L4440 (left column, top row). Inset, one entire muscle cell depicted. Scale bar = 10 μm. N = 50 nematodes per condition. (E) Western blots of the supernatant and pellet profiles of luciR188Q,R216Q‐YFP in lysates obtained from single and double RNAi knockdown (KD) of cytosolic and nuclear class A and class B J‐proteins in muscle tissue of C. elegans. The YFP moiety fused to luciR188Q,R216Q immunoblotted with a cross‐reacting antibody raised against GFP.
