Abstract
The aim of this review is to analyze what’s new on anesthetic prospective to perioperative management for thyroid surgery. For recent decades intraoperative neuromonitoring (IONM) during thyroid and parathyroid surgery has obtained more and more popularity. New modality of anesthetic technique was also developed to incorporate into surgical teamwork. For example, the precise position of EMG tube and optimal use of neuromuscular blocking agents (NMBAs) play key roles in successful IONM system. Special focus is paid to following issues: (I) preoperative airway evaluation and pre-op preparation; (II) anesthetic managements including advanced intubation tools, NMBAs and sugammadex; and (III) post-op adverse events such as pain and postoperative nausea vomiting.
Keywords: Thyroid surgery, anesthesia, intraoperative neuromonitoring (IONM)
Introduction
Traditional roles of anesthesia to thyroidectomy include preoperative assessment of thyroid function, anticipated difficult airway, adequate surgical relaxation and postoperative urgent airway complications (hematoma, bilateral vocal palsy) (1,2). In past decades, intraoperative neuromonitoring (IONM) has been widely accepted as adjunct technique to identify the target nerves, to detect variation, to elucidate nerve injury mechanism and to assess real-time nerve function during thyroid surgery (3-9). Therefore, special considerations should be taken into anesthetic management to ensure anesthesia-related factors to successful IONM system. For example, malposition of electromyography (EMG) tube and improper use of neuromuscular blocking agent (NMBA) might be the common causes of monitor dysfunction. Worldwide standard IONM protocol or international guideline has been published to make neural monitor as a precise and certain system for thyroid surgery (10). This review is intended to analyze what’s new on anesthetic prospective to improve the quality of care and to reduce perioperative adverse events.
Preoperative evaluation
Upper airway evaluation (including denture)
Tracheal intubation is the key technique of general anesthesia. However, this technique was associated with various complications such as tissue trauma particularly when difficult intubation occurred. In the literatures, thyroid surgery is considered as a risk factor for difficult intubation due to large goiter or cancer. The reported incidence of difficult intubation in thyroid surgery ranged from 5.3% to 24.6% which was higher than general population (11-13). A detailed upper airway assessment is a necessary part of preoperative evaluation. In our medical center at Taiwan, an online electronic system of preoperative evaluation was designed to ensure complete upper airway and dental assessment (Figure 1). The online preoperative evaluation system could not progress without fulfilling airway and dental requested parameters. A detailed and careful preoperative assessment of upper airway might predict difficult intubation and alert potential dental injury to anesthesiologists. Kuo et al. reported that anesthesia-related dental trauma was significantly reduced after a quality improvement program based on routine dental diagram and difficult airway parameters of every patient undergoing elective surgery (14).
Figure 1.
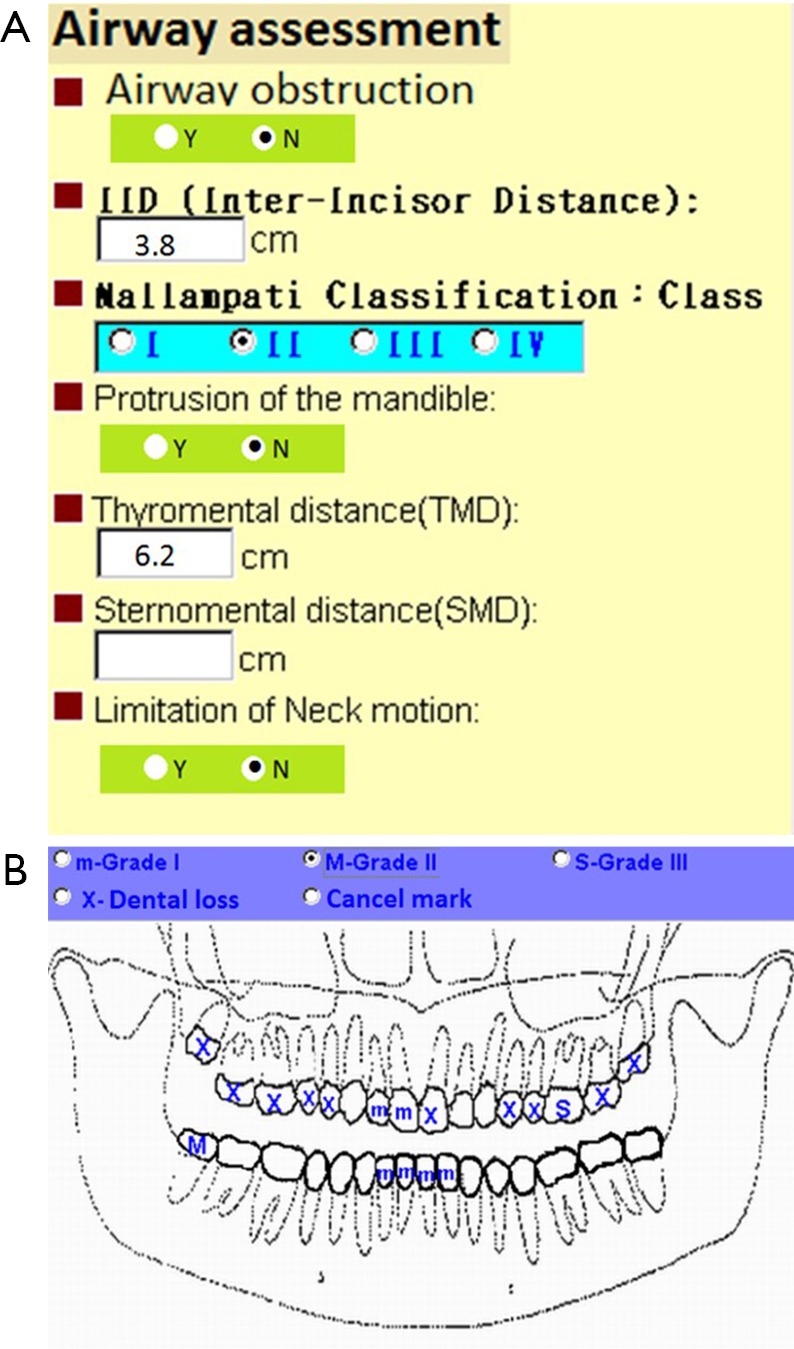
A sample of airway and dental assessment of electronic preoperative evaluation system. (A) Routine airway document to screen possible difficult intubation; (B) standardized electronic dental chart to record dental loss or teeth mobility (m = mild, M = moderate, S = severe).
Preoperative preparation following enhanced recovery after surgery (ERAS) protocols
ERAS protocols or the perioperative surgical home (PSH) has become an important issue of care coordination to improve medical outcome. In recent years, anesthesiologists play a key role in those perioperative care coordination (15,16). ERAS protocols consist of multimodal perioperative managements developed to reduce complications and length of stay after surgery by maintenance of preoperative organ function and reduction of postoperative stress response (17). The shortening of preoperative fasting has proposed to replace fasting patient from the midnight without increase in aspiration risk (18). Preoperative solid food up to 6 hours and clear fluids or clear carbohydrate drinks up to 2 hours were allowed to attenuate insulin resistance, protein loss, muscle wasting, hunger, thirst and anxiety (19-22). Preadmission counseling, antibiotic prophylaxis, no premedication and thromboprophylaxis were also recommended as preoperative preparation for major surgery in ERAS protocols (23).
Anesthetic management
Advanced airway devices and EMG tube positioning
As mentioned previously, the incidence of difficult intubation may be higher in thyroid surgery when conventional intubation tool “laryngoscope” was used. During thyroid surgery with neuromonitoring setting, the success of airway management demands not only correct tracheal intubation but also proper position of EMG tube (24,25). Accurate positioning of the EMG endotracheal tube is the key step for the precise neuromonitoring. Kim et al. used a porcine model to demonstrate that artificial endotracheal tube rotation and depth changes induced significant EMG amplitude change with relatively stable EMG latency (26). In a retrospective review of ten patients with EMG tube malposition, similar EMG outcomes (decrease in EMG amplitudes but not latency) were found during tube rotation or vertical displacement (27). Cherng et al. showed tube rotation caused by the right or left side mouth corner fixations in a manikin study; therefore, middle fixation of EMG tube between incisors was recommended for IONM setup (28).
In recent decades, many novel airway devices have been designed to improve success rate of tracheal intubation and reduce intubation difficulty (29-31). There are two major system of advanced airway device: (I) video-assisted laryngoscope with a changeable blade such as the GlideScope, the Airway Scope (AWS; Pentax) and UESCOPE. (II) Intubating stylet with a rigid fiberscope for direct visualization such as Trachway Video Intubating stylet (Trachway), and the Bonfils fiberscope. When an EMG tube placement is requested for neuromonitoring, the GlideScope not only allows direct visualization of glottis but also enables proper surface electrode positioning to the vocal cords during tube placement (32,33). The EMG tube could be placed via the Trachway (Figure 2) and further rechecked position under fiberscopic visualization after successful tracheal intubation.
Figure 2.
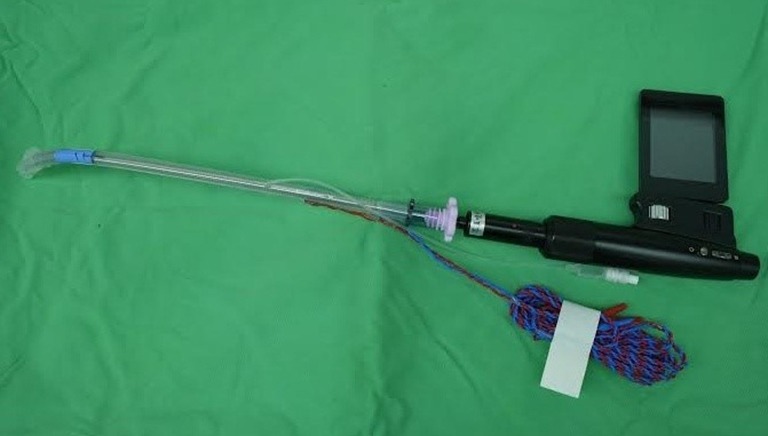
The Trachway intubating stylet loaded with an EMG endotracheal tube. The rigid stylet was lengthened up to 34 cm fit for the EMG tube. EMG, electromyography.
NMBA and sugammadex
In anesthetic perspective, NMBAs (muscle relaxants) is generally considered as gold standard for surgical relaxation and tracheal intubation with less airway complications (34,35). In neuromonitoring era, the liberal use of NMBA might diminish derived EMG signals to various degrees and confuse interpretation of IONM results (36-40). A series of porcine models and clinical trials have proceeded to investigate suitable non-depolarizing NMBA and optimal dose feasible to IONM during thyroid surgery.
There are five strategies of neuromuscular blockade management for IONM as followings: (I) no NMBA use for entire perioperative period. It is possible to perform tracheal intubation without muscle relaxant with experienced hands (41). However, it was not suggested as a routine practice due to higher risk of airway injury (34,35). (II) Depolarizing NMBA (succinylcholine). Succinyl choline at 2 to 2.5 mg per kilogram is an ideal choice with respect to rapid onset and short duration of muscular relaxation (10). Many anesthesiologists avoid succinyl choline because of its side effects, such as cardiac dysrhythmia, hyperkalemia, and malignant hyperthermia (42,43). (III) Single induction dose of non-depolarizing NMBA. For example, rocuronium and atracurium at 0.5 mg/kg was adequate for tracheal intubation and allowed spontaneous recovery of neuromuscular transmission and positive EMG signals gradually (44). (IV) Single reduced dose of rocuronium. One of widely accepted regimen was one effective dose of rocuronium (0.3 mg/kg) for anesthesia induction. It was recommended as an optimal dose for IONM during thyroid surgery for providing high EMG amplitude at early stage of operation and satisfactory intubating conditions (36). (V) Two effective dose of rocuronium with subsequent sugammadex. Rocuronium 0.6 mg/kg at anesthesia induction yields excellent intubating condition. Sugammadex acts as a selective relaxant binding agent for rapid neuromuscular blockade reversal induced by steroidal NMBA (i.e., rocuronium, vecuronium) (45,46). Sugammadex 2 mg/kg at skin incision rapidly restores neuromuscular function suppressed by rocuronium. The regimen seems to meet both anesthesia (intubation) and surgery (monitoring) demands. The drawback is the high cost of sugammadex which restricts its use in most countries currently (47).
Post-operative adverse events
Pain-multimodal analgesia
Postoperative pain control by multimodal analgesia using more than one analgesic modality is mandatory to enhanced recovery. Undermanaged postoperative pain delays recovery and discharge after surgery (48). Parenteral opioids are effective analgesic regimen but are associated with adverse effects such as respiratory depression, postoperative nausea and vomiting (PONV), pruritus, urinary retention, and ileus. Postoperative recovery and hospital stay may prolong if those unwanted side effects occur (49). Advancements in multimodal analgesic techniques are aimed to achieve more effective pain control and less opioid-related side effects (50).
Available systemic analgesia except opioids includes followings: non-steroidal anti-inflammatory drugs (NSAID) and cyclooxygenase-2 (COX-2) inhibitor (parecoxib), acetaminophen (oral or intravenous), alpha 2 agonists (clonidine or dexmedetomidine),intravenous lidocaine infusion, anticonvulsants (gabapentin and pregabalin), glucocorticoids (e.g., dexamethasone), beta-blockers (e.g., esmolol), N-methyl-D-aspartate (NMDA) receptor antagonists (ketamine) (51). Parecoxib, the only intravenous COX-2 inhibitor, has been reported to reduce opioid consumption in major surgery (total knee replacement) (52) and to provide comparable analgesic effect as opioids in minor surgery (laryngeal microsurgery) (53). Furthermore, fewer opioid-related adverse events and faster functional recovery could be expected.
PONV
It has been reported that PONV may prolong the recovery, delay discharge from hospital and reduce patient satisfaction (54). PONV affects 25–30% of overall surgical population (54,55) and the incidence becomes as high as 63–84% in patients undergoing thyroid surgery (56-58). The etiology of PONV is complex and can be classified into patient, anesthetic and surgical factors. The incidence might be predicted by following risk factors: a history of motion sickness or PONV, female patients, non-smokers, postoperative opioids use and emetogenic surgery (59,60). A multimodal approach to PONV prophylaxis by more than two interventions is suggested in adults with high risk (>2 risk factors) (54,61).
Prophylaxis and treatment interventions included propofol anesthesia, propofol low dose infusion, traditional antiemetics (droperidol, dimenhydrinate, scopolamine, metoclopramide), non-traditional antiemetics (propofol and dexamethasone), and serotonin receptor antagonist (ondansetron) and non-pharmacologic approach such as acupuncture (61,62). If prophylaxis fails and PONV is present, a multimodal approach is recommended by using antiemetic from different class than prophylactic drug. Alternatively, propofol anesthesia alone or combined with inhaled anesthetics can be beneficial in patients with susceptibility to PONV. Chen et al. reported a relative low PONV incidence (3.8–4.2%) with propofol target-controlled infusion in patients undergoing laparoscopic cholecystectomy (63).
Conclusions
There have been many efforts paid to optimize anesthetic care for thyroid surgery, particularly with specific perioperative management to facilitate IONM protocols. Perioperative managements proposed to enhance recovery after surgery may be beneficial to thyroid surgery but it requires further evidence. Airway management should be prepared for any possible airway difficulty and proper position of the endotracheal tube. IONM has become a mature adjuvant for a possible event of any nerve injuries and palsies. A well communication between surgeons and anesthesiologists will avoid potential obstacles and interferences to neuromonitoring setup. Adequate prophylaxis and treatment of pain and PONV are of major concern during postoperative care. Currently, anesthesiologists not merely serve as an isolated guardian to keep patient safety but also as a part of teamwork to enhance better medical outcomes.
Acknowledgements
Funding: This study was supported by grants from the Kaohsiung Medical University (KMUH105-5R39; KMU-TP105E23) and the Ministry of Science and Technology (MOST 105-2314-B-037-010) Taiwan.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bajwa SJ, Sehgal V. Anesthesia and thyroid surgery: The never ending challenges. Indian J Endocrinol Metab 2013;17:228-34. 10.4103/2230-8210.109671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacuzzi A, Dionigi G, Del Bosco A, et al. Anaesthesia for thyroid surgery: perioperative management. Int J Surg 2008;6 Suppl 1:S82-5. 10.1016/j.ijsu.2008.12.013 [DOI] [PubMed] [Google Scholar]
- 3.Chiang FY, Lee KW, Chen HC, et al. Standardization of intraoperative neuromonitoring of recurrent laryngeal nerve in thyroid operation. World J Surg 2010;34:223-9. 10.1007/s00268-009-0316-8 [DOI] [PubMed] [Google Scholar]
- 4.Dralle H, Sekulla C, Lorenz K, et al. Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg 2008;32:1358-66. 10.1007/s00268-008-9483-2 [DOI] [PubMed] [Google Scholar]
- 5.Chiang FY, Lu IC, Kuo WR, et al. The mechanism of recurrent laryngeal nerve injury during thyroid surgery--the application of intraoperative neuromonitoring. Surgery 2008;143:743-9. 10.1016/j.surg.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 6.Thomusch O, Sekulla C, Machens A, et al. Validity of intra-operative neuromonitoring signals in thyroid surgery. Langenbecks Arch Surg 2004;389:499-503. 10.1007/s00423-003-0444-9 [DOI] [PubMed] [Google Scholar]
- 7.Hermann M, Hellebart C, Freissmuth M. Neuromonitoring in thyroid surgery: prospective evaluation of intraoperative electrophysiological responses for the prediction of recurrent laryngeal nerve injury. Ann Surg 2004;240:9-17. 10.1097/01.sla.0000132260.34503.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang FY, Lu IC, Chen HC, et al. Intraoperative neuromonitoring for early localization and identification of recurrent laryngeal nerve during thyroid surgery. Kaohsiung J Med Sci 2010;26:633-9. 10.1016/S1607-551X(10)70097-8 [DOI] [PubMed] [Google Scholar]
- 9.Chiang FY, Lu IC, Chen HC, et al. Anatomical variations of recurrent laryngeal nerve during thyroid surgery: how to identify and handle the variations with intraoperative neuromonitoring. Kaohsiung J Med Sci 2010;26:575-83. 10.1016/S1607-551X(10)70089-9 [DOI] [PubMed] [Google Scholar]
- 10.Randolph GW, Dralle H, International Intraoperative Monitoring Study G, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121 Suppl 1:S1-16. 10.1002/lary.21119 [DOI] [PubMed] [Google Scholar]
- 11.Kalezić N, Milosavljevic R, Paunovic I, et al. The incidence of difficult intubation in 2000 patients undergoing thyroid surgery--a single center expirience. Vojnosanit Pregl 2009;66:377-82. 10.2298/VSP0905377K [DOI] [PubMed] [Google Scholar]
- 12.Amathieu R, Smail N, Catineau J, et al. Difficult intubation in thyroid surgery: myth or reality? Anesth Analg 2006;103:965-8. 10.1213/01.ane.0000237305.02465.ee [DOI] [PubMed] [Google Scholar]
- 13.Bouaggad A, Nejmi SE, Bouderka MA, et al. Prediction of difficult tracheal intubation in thyroid surgery. Anesth Analg 2004;99:603-6, table of contents. 10.1213/01.ANE.0000122634.69923.67 [DOI] [PubMed] [Google Scholar]
- 14.Kuo YW, Lu IC, Yang HY, et al. Quality improvement program reduces perioperative dental injuries - A review of 64,718 anesthetic patients. J Chin Med Assoc 2016;79:678-82. 10.1016/j.jcma.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Schweitzer M. The perioperative surgical home: what anesthesiologists need to do. J Med Pract Manage 2014;29:235-7. [PubMed] [Google Scholar]
- 16.Holt NF. Trends in healthcare and the role of the anesthesiologist in the perioperative surgical home - the US perspective. Curr Opin Anaesthesiol 2014;27:371-6. 10.1097/ACO.0000000000000064 [DOI] [PubMed] [Google Scholar]
- 17.Albalawi Z, Laffin M, Gramlich L, et al. Enhanced Recovery After Surgery (ERAS®) in Individuals with Diabetes: A Systematic Review. World J Surg 2017;41:1927-34. 10.1007/s00268-017-3982-y [DOI] [PubMed] [Google Scholar]
- 18.Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev 2003;(4):CD004423. [DOI] [PubMed] [Google Scholar]
- 19.Svanfeldt M, Thorell A, Hausel J, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg 2007;94:1342-50. 10.1002/bjs.5919 [DOI] [PubMed] [Google Scholar]
- 20.Soop M, Nygren J, Thorell A, et al. Preoperative oral carbohydrate treatment attenuates endogenous glucose release 3 days after surgery. Clin Nutr 2004;23:733-41. 10.1016/j.clnu.2003.12.007 [DOI] [PubMed] [Google Scholar]
- 21.Soop M, Carlson GL, Hopkinson J, et al. Randomized clinical trial of the effects of immediate enteral nutrition on metabolic responses to major colorectal surgery in an enhanced recovery protocol. Br J Surg 2004;91:1138-45. 10.1002/bjs.4642 [DOI] [PubMed] [Google Scholar]
- 22.Nygren J, Soop M, Thorell A, et al. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clin Nutr 1998;17:65-71. 10.1016/S0261-5614(98)80307-5 [DOI] [PubMed] [Google Scholar]
- 23.Melnyk M, Casey RG, Black P, et al. Enhanced recovery after surgery (ERAS) protocols: Time to change practice? Can Urol Assoc J 2011;5:342-8. 10.5489/cuaj.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai CJ, Tseng KY, Wang FY, et al. Electromyographic endotracheal tube placement during thyroid surgery in neuromonitoring of recurrent laryngeal nerve. Kaohsiung J Med Sci 2011;27:96-101. 10.1016/j.kjms.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 25.Lu IC, Chu KS, Tsai CJ, et al. Optimal depth of NIM EMG endotracheal tube for intraoperative neuromonitoring of the recurrent laryngeal nerve during thyroidectomy. World J Surg 2008;32:1935-9. 10.1007/s00268-008-9549-1 [DOI] [PubMed] [Google Scholar]
- 26.Kim HY, Tufano RP, Randolph G, et al. Impact of positional changes in neural monitoring endotracheal tube on amplitude and latency of electromyographic response in monitored thyroid surgery: Results from the Porcine Experiment. Head Neck 2016;38 Suppl 1:E1004-8. 10.1002/hed.24145 [DOI] [PubMed] [Google Scholar]
- 27.Barber SR, Liddy W, Kyriazidis N, et al. Changes in electromyographic amplitudes but not latencies occur with endotracheal tube malpositioning during intraoperative monitoring for thyroid surgery: Implications for guidelines. Laryngoscope 2017;127:2182-8. 10.1002/lary.26392 [DOI] [PubMed] [Google Scholar]
- 28.Cherng CH, Huang YH, Shih ML. Middle fixation of electromyographic endotracheal tube for intraoperative recurrent laryngeal nerve monitoring. J Clin Anesth 2014;26:252-3. 10.1016/j.jclinane.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 29.Tseng KY, Chau SW, Su MP, et al. A comparison of Trachway intubating stylet and Airway Scope for tracheal intubation by novice operators: a manikin study. Kaohsiung J Med Sci 2012;28:448-51. 10.1016/j.kjms.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 30.Nouruzi-Sedeh P, Schumann M, Groeben H. Laryngoscopy via Macintosh blade versus GlideScope: success rate and time for endotracheal intubation in untrained medical personnel. Anesthesiology 2009;110:32-7. 10.1097/ALN.0b013e318190b6a7 [DOI] [PubMed] [Google Scholar]
- 31.Sun DA, Warriner CB, Parsons DG, et al. The GlideScope Video Laryngoscope: randomized clinical trial in 200 patients. Br J Anaesth 2005;94:381-4. 10.1093/bja/aei041 [DOI] [PubMed] [Google Scholar]
- 32.Kanotra SP, Kuriloff DB, Lesser J, et al. GlideScope-assisted nerve integrity monitoring tube placement for intra-operative recurrent laryngeal nerve monitoring. J Laryngol Otol 2012;126:1271-3. 10.1017/S0022215112002460 [DOI] [PubMed] [Google Scholar]
- 33.Berkow L, Dackiw AP, Tufano RP. Use of the GlideScope for placement of a recurrent laryngeal nerve monitoring endotracheal tube. J Clin Anesth 2011;23:81-3. 10.1016/j.jclinane.2009.12.013 [DOI] [PubMed] [Google Scholar]
- 34.Mencke T, Knoll H, Schreiber JU, et al. Rocuronium is not associated with more vocal cord injuries than succinylcholine after rapid-sequence induction: a randomized, prospective, controlled trial. Anesth Analg 2006;102:943-9. 10.1213/01.ane.0000194509.03916.02 [DOI] [PubMed] [Google Scholar]
- 35.Mencke T, Echternach M, Kleinschmidt S, et al. Laryngeal morbidity and quality of tracheal intubation: a randomized controlled trial. Anesthesiology 2003;98:1049-56. 10.1097/00000542-200305000-00005 [DOI] [PubMed] [Google Scholar]
- 36.Lu IC, Tsai CJ, Wu CW, et al. A comparative study between 1 and 2 effective doses of rocuronium for intraoperative neuromonitoring during thyroid surgery. Surgery 2011;149:543-8. 10.1016/j.surg.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Hou V, Tucker A, et al. Changes of extremity and laryngeal muscle electromyographic amplitudes after intravenous administration of vecuronium. Laryngoscope 2008;118:2156-60. 10.1097/MLG.0b013e318184574e [DOI] [PubMed] [Google Scholar]
- 38.Marusch F, Hussock J, Haring G, et al. Influence of muscle relaxation on neuromonitoring of the recurrent laryngeal nerve during thyroid surgery. Br J Anaesth 2005;94:596-600. 10.1093/bja/aei110 [DOI] [PubMed] [Google Scholar]
- 39.Debaene B, Plaud B, Dilly MP, et al. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology 2003;98:1042-8. 10.1097/00000542-200305000-00004 [DOI] [PubMed] [Google Scholar]
- 40.Chu KS, Tsai CJ, Lu IC, et al. Influence of nondepolarizing muscle relaxants on intraoperative neuromonitoring during thyroid surgery. J Otolaryngol Head Neck Surg 2010;39:397-402. [PubMed] [Google Scholar]
- 41.Hanci V, Erdogan G, Okyay RD, et al. Effects of fentanyl-lidocaine-propofol and dexmedetomidine-lidocaine-propofol on tracheal intubation without use of muscle relaxants. Kaohsiung J Med Sci 2010;26:244-50. 10.1016/S1607-551X(10)70035-8 [DOI] [PubMed] [Google Scholar]
- 42.Miller R. Will succinylcholine ever disappear? Anesth Analg 2004;98:1674-5. 10.1213/01.ANE.0000126935.87196.4C [DOI] [PubMed] [Google Scholar]
- 43.Orebaugh SL. Succinylcholine: adverse effects and alternatives in emergency medicine. Am J Emerg Med 1999;17:715-21. 10.1016/S0735-6757(99)90168-7 [DOI] [PubMed] [Google Scholar]
- 44.Chu KS, Wu SH, Lu IC, et al. Feasibility of intraoperative neuromonitoring during thyroid surgery after administration of nondepolarizing neuromuscular blocking agents. World J Surg 2009;33:1408-13. 10.1007/s00268-009-0049-8 [DOI] [PubMed] [Google Scholar]
- 45.Welliver M, McDonough J, Kalynych N, et al. Discovery, development, and clinical application of sugammadex sodium, a selective relaxant binding agent. Drug Des Devel Ther 2009;2:49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorgenfrei IF, Norrild K, Larsen PB, et al. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: a dose-finding and safety study. Anesthesiology 2006;104:667-74. 10.1097/00000542-200604000-00009 [DOI] [PubMed] [Google Scholar]
- 47.Lu IC, Wu CW, Chang PY, et al. Reversal of rocuronium-induced neuromuscular blockade by sugammadex allows for optimization of neural monitoring of the recurrent laryngeal nerve. Laryngoscope 2016;126:1014-9. 10.1002/lary.25577 [DOI] [PubMed] [Google Scholar]
- 48.American Society of Anesthesiologists Task Force on Acute Pain Management Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012;116:248-73. 10.1097/ALN.0b013e31823c1030 [DOI] [PubMed] [Google Scholar]
- 49.Oderda GM, Evans RS, Lloyd J, et al. Cost of opioid-related adverse drug events in surgical patients. J Pain Symptom Manage 2003;25:276-83. 10.1016/S0885-3924(02)00691-7 [DOI] [PubMed] [Google Scholar]
- 50.Kehlet H, Dahl JB. The value of "multimodal" or "balanced analgesia" in postoperative pain treatment. Anesth Analg 1993;77:1048-56. [DOI] [PubMed] [Google Scholar]
- 51.Tan M, Law LS, Gan TJ. Optimizing pain management to facilitate Enhanced Recovery After Surgery pathways. Can J Anaesth 2015;62:203-18. 10.1007/s12630-014-0275-x [DOI] [PubMed] [Google Scholar]
- 52.Buvanendran A, Kroin JS, Tuman KJ, et al. Effects of perioperative administration of a selective cyclooxygenase 2 inhibitor on pain management and recovery of function after knee replacement: a randomized controlled trial. JAMA 2003;290:2411-8. 10.1001/jama.290.18.2411 [DOI] [PubMed] [Google Scholar]
- 53.Huang HF, Chang PY, Chen YC, et al. Single bolus parecoxib attenuates sore throat after laryngeal microsurgery: a randomized double-blind control study. Kaohsiung J Med Sci 2014;30:574-8. 10.1016/j.kjms.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 54.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg 2013;37:259-84. 10.1007/s00268-012-1772-0 [DOI] [PubMed] [Google Scholar]
- 55.Matsuura H, Inoue S, Kawaguchi M. The risk of postoperative nausea and vomiting between surgical patients received propofol and sevoflurane anesthesia: A matched study. Acta Anaesthesiol Taiwan 2016;54:114-20. 10.1016/j.aat.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 56.Sonner JM, Hynson JM, Clark O, et al. Nausea and vomiting following thyroid and parathyroid surgery. J Clin Anesth 1997;9:398-402. 10.1016/S0952-8180(97)00069-X [DOI] [PubMed] [Google Scholar]
- 57.Ewalenko P, Janny S, Dejonckheere M, et al. Antiemetic effect of subhypnotic doses of propofol after thyroidectomy. Br J Anaesth 1996;77:463-7. 10.1093/bja/77.4.463 [DOI] [PubMed] [Google Scholar]
- 58.Dejonckheere M, Deloof T, Dustin N, et al. Alizapride in the Prevention of Post-Thyroidectomy Emetic Sequelae. Eur J Anaesth 1990;7:421-7. [Google Scholar]
- 59.Apfel CC, Kranke P, Katz MH, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth 2002;88:659-68. 10.1093/bja/88.5.659 [DOI] [PubMed] [Google Scholar]
- 60.Apfel CC, Laara E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999;91:693-700. 10.1097/00000542-199909000-00022 [DOI] [PubMed] [Google Scholar]
- 61.Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2014;118:85-113. 10.1213/ANE.0000000000000002 [DOI] [PubMed] [Google Scholar]
- 62.Fujii Y. The benefits and risks of different therapies in preventing postoperative nausea and vomiting in patients undergoing thyroid surgery. Curr Drug Saf 2008;3:27-34. 10.2174/157488608783333934 [DOI] [PubMed] [Google Scholar]
- 63.Chen PN, Lu IC, Chen HM, et al. Desflurane reinforces the efficacy of propofol target-controlled infusion in patients undergoing laparoscopic cholecystectomy. Kaohsiung J Med Sci 2016;32:32-7. 10.1016/j.kjms.2015.11.006 [DOI] [PubMed] [Google Scholar]


