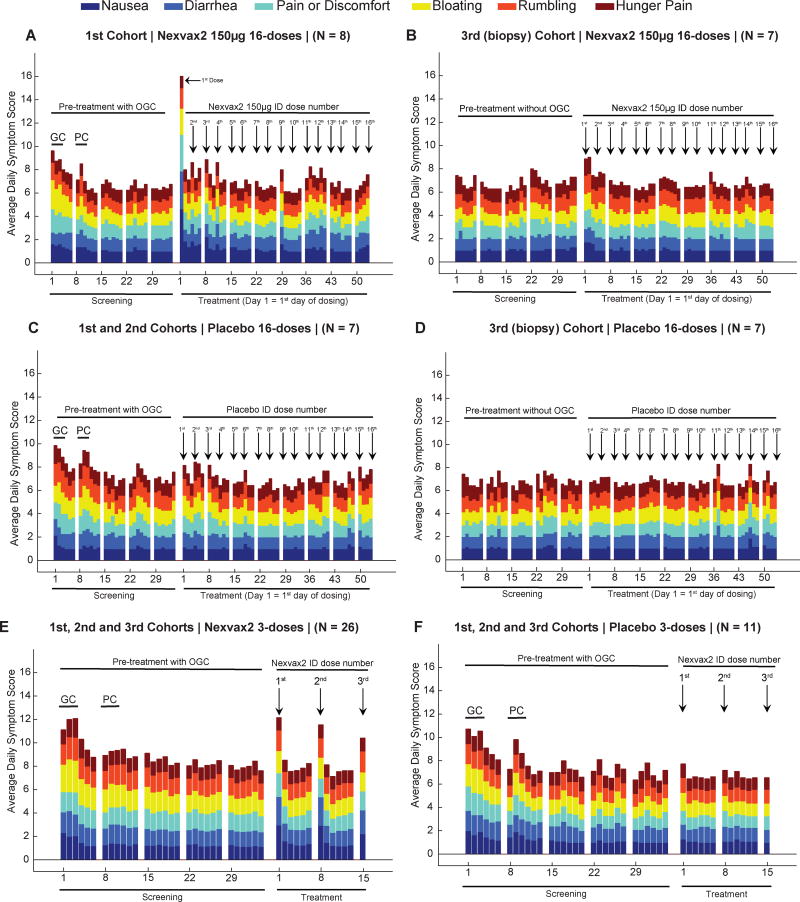
Figure 3. Gastrointestinal symptoms.
Participants scored six items in the Gastrointestinal Symptoms Rating Scale (GSRS) from 1 (no discomfort at all) to 6 (very severe discomfort) every day except the last day of each week during the 16-dose (A–D), and 3-dose studies (E and F). In ascending dose cohorts (A, C, E and F), 3-day gluten challenge (GC) corresponds to Screening days 1 to 3, and placebo challenge (PC) corresponds to Screening days 8 to 10; the biopsy cohorts did not have a gluten challenge (B and D). The sum of six symptom scores increased when Nexvax2 was first administered (Treatment day 1) and reached statistical significance in participants receiving Nexvax2 150µg (A) compared to placebo (C) in ascending dose cohorts of the 16-dose study (P=0·015; Wilcoxon rank sum test).