Abstract
Background
Disease patterns in Mexican-American health-disparity populations differ from larger United States populations.
Aims
Determine frequency of gastrointestinal cancers in Mexican Americans.
Methods
We analyzed self-reported data from the Cameron County Hispanic Cohort where we find high rates of risk factors for cancer: obesity (48.5%), diabetes (30.7%). Participants provided cancer histories about themselves and first and second degree relatives. Logistic regression models assessed risk factors. Frequencies of cancer sites were ranked and validated using concurrent age local cancer registry data.
Results
Among 9,249 individuals (participants and their relatives) there were 1,184 individuals with reports of cancer. Among cohort participants under 70 years of age the most significant risk factor for all-cause cancers was diabetes (OR 3.57, 95% CI 1.32, 9.62). Participants with metabolic syndrome were significantly more likely to report cancer in relatives (1.73 (95%CI 1.26, 2.37). Among cancers in fathers, liver cancer was ranked third, stomach fourth, colorectal sixth and pancreas tenth. In mothers, stomach was third, liver fourth, colorectal seventh and pancreas eleventh. The unusual prominence of these cancers in Mexican Americans, including liver cancer, was supported by age-adjusted incidence in local registry data.
Conclusions
Gastrointestinal system cancers, particularly liver cancer, in a Mexican American health disparity cohort and their relatives rank higher than in other ethnicities and are associated with high rates of diabetes and metabolic syndrome. Effective prevention of diabetes and low-tech, high-quality screening strategies for gastrointestinal cancers are needed in health disparity communities.
Keywords: gastrointestinal cancer, liver, diabetes, Mexican Americans
Background & Aims
Cancer is a major contributor to the pandemic of non-communicable diseases (NCDs) together with cardiovascular diseases, chronic lung disease and diabetes.(1) The World Health Organization (WHO) in 2014 estimated the global burden of new cancer cases will rise from the current 14 million annually to 22 million by the 2040s, when it will likely account for 13 million deaths each year.(2) Obesity and diabetes have been recently linked to increased cancer incidence and mortality, particularly cancers of the gastrointestinal system and breast.(3,4) Though much emphasis has been placed on cancers of the lung, breast and prostate, the increase in gastrointestinal cancers may have been under-appreciated.
In the United States (U.S.) the Hispanic population is estimated to account for 30% of the population by the year 2020.(5) However, sub-populations vary and their origin is not always distinguished.(6,7) The largest sub-group (64%) is Mexican-American, among whom diabetes-related chronic diseases are prominent and where severe socioeconomic and health disparities are similar to those found globally in low-and middle-income countries (LMICs) with high NCD prevalence.(6,8-11) Cancer (21.1%) has now surpassed heart disease (20.9%) as a leading cause of death among Hispanics.(12) However, estimates suffer from underreporting.(13-16) The unique socioeconomic, age distribution and immigration histories of Hispanics together with high rates of poverty and lack of insurance may account for much of these differences.(12)
Hispanics are more likely to present with advanced cancers and have lower five-year survival rates.(13) Mexican-Americans also experience lower screening rates for essentially preventable or treatable cancers than any other ethnic group.(6,13)
These disparities are more pronounced along the US/Mexico border than in any other region of the U.S. such that accurate data on cancer-specific rates are difficult to ascertain.(17) Age-adjusted rates of diabetes are higher in Hispanics than in non-Hispanics in the U.S., with Mexican-Americans having among the highest incidence and mortality rates.(18-21) Along the U.S./Mexico border Mexican Americans have 30.7% prevalence of diabetes and 49.5% for obesity.(22) In this environment as in LMICs there is little reliable mortality data and weak surveillance systems.(1) Data from cancer registries, however, do suggest elevated incidence of liver cancers in this population.(23) On the U.S./Mexico border the majority of reports are from hospitals but since two thirds of the population has no health insurance many cases are not recorded and cancer registry data are limited. Such patients often seek terminal care elsewhere, frequently in adjacent Mexico.(11,24) Our objective was to estimate the burden and frequency of cancers in an extensively documented Mexican American community cohort living in socioeconomic conditions similar to many LMICs to provide insight into the impact of gastrointestinal cancers nationally and globally.(11,25)
Methods
Study Design
We analyzed cross-sectional community-based data from participants in our Cameron County Hispanic Cohort (CCHC). For the purposes of this study, the main outcome measures were self-reported cancers in participants and their first and second degree relatives. The CCHC now numbers over 3,000 individuals recruited from their households in Brownsville on the US/Mexico border in one of the two poorest counties in the United States.(10) Recruiting began in 2004 and is ongoing with 5 and 10 year follow-up in hand. This cohort has provided extensive data showing weighted prevalence of a range of diseases in this health disparity community. Questionnaires are administered in English or Spanish, with about 70% of the participants electing Spanish.
IRB Approval
All protocols, procedures and consent forms were reviewed and approved by The University of Texas Health Science Center, Committee for the Protection of Human Subjects (HSC-SPH-03-007-B).
Setting and Data Collection
CCHC participants aged 18 years and older are recruited using two-stage randomized sampling of households in the city of Brownsville, Texas.(25) After obtaining written informed consent, extensive socio-demographic and medical histories including cancers are collected in our Clinical Research Unit in the language of choice of the participants. Each participant is also asked about history of cancers in first (parents and siblings) and second (grandparents and parents' siblings) degree relatives. We measured diabetes status (fasting blood glucose and glycosylated hemoglobin), height, weight, lipid profile and fasting insulin. Body Mass Index (BMI) was calculated from kg/m2 and insulin resistance using the homeostasis model assessment equation (HOMA-IR).(26) For first and second degree relatives we used participant-reported data, which we call “participant-reported” for simplicity. For parents of participants, data were limited to diabetes status and country of birth. For other relatives no data other than occurrence of cancer were collected.
Statistical analysis
All data were analyzed using SAS software version 9.2 TS level 1MO (SAS Institute Inc., Cary, NC). All hypotheses were tested at the 0.05 level of significance.
Participants
We used sampling weights as previously described.(25) Descriptive statistics examined sociodemographic and biological risk factors using a design-based analysis incorporating age- and gender-adjusted sampling weights, stratification by socioeconomic status and allowing for clustering by census block and household. Weighted odds ratios and their 95% confidence intervals are presented. We took all variables in the univariable analysis with p values less than 0.25 and assessed their significance in a multivariable weighted logistic regression model controlling for age and gender.
First and second degree relatives
We assessed the proportion of all cancer events among parents by major cancer sites and by gender, and association with the parent's diabetes status and country of birth. Data from parents were then ranked by individual cancer relative frequencies.
Comparison with registry data
To validate our observations we compared our findings to public and published data available for Hispanics and non-Hispanics across the nation, for Hispanics in Texas and data from Mexico.(27-30) Finally we reviewed cancer site incidence by ethnic origin using Texas Cancer Registry Data.(28) (Texas cancer data provided by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, 211 E. 7th Street, Suite 325, Austin, TX 78701).
Results
Overall reports of cancer events
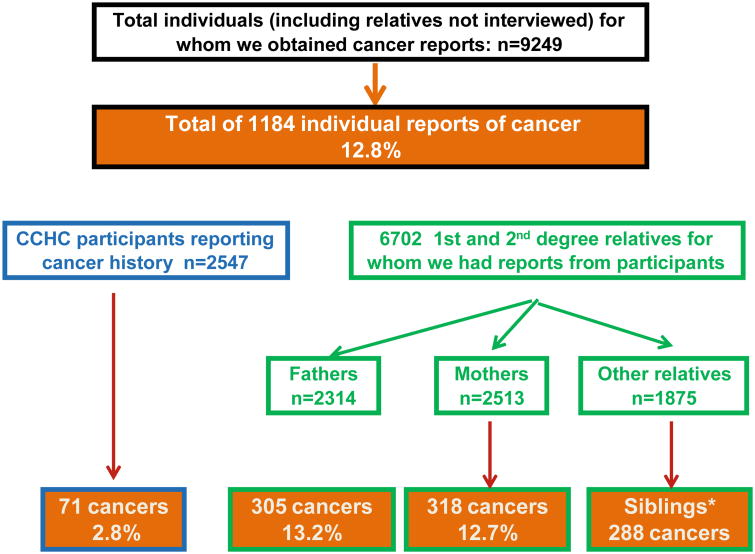
The figure shows that there were 1,184 records of cancer from 9,249 reports on individuals (alive or dead) given us by cohort participants (12.8%); 2,547 participants, 6,702 first and second degree relatives (2,314 fathers, 2,513 mothers and 1,875 other close relatives). Seventy one personal cancer histories at the time of questioning were elicited from the participants (2.8%). In first degree relatives there were 305 reports of all cause cancers in fathers (13.2%), 318 in mothers (12.7%) and 288 in siblings. In second degree relatives there were 202 reports of all cause cancers.
Fig. 1. Algorithm showing the numbers of participants and their relatives on whom we had cancer data, and the number and frequencies of all cause cancers in the groups.
Participants
Socio-demographic and health characteristics of the 2,547participants and their family histories of cancer are shown in table 1. More than half of all participants were born in Mexico, the majority living below the poverty level, with high unemployment. In a previous analysis we found that more than two thirds of cohort participants were uninsured.(22) The weighted prevalence of all cause cancer was 4.2%. Diabetes was a highly significant risk factor (OR 2.62, 95%CI 1.19, 5.79). Not surprisingly, older age and smoking were also risk factors. Because of the limited sample size and timescale of the cohort itself for cancer endpoints, numbers of reported cancers for individual sites in participants were too small for separate analyses.
Table 1. Bivariate analysis of weighted demographic and biological variables for reported cancer in participants, Cameron County Hispanic Cohort, 2000–2011.
| Total (n=2547) | With Cancer (n=71) | Odds Ratio for cancer (95% CI) | |
|---|---|---|---|
| Age (years) | n* | n (%)* | OR (95%CI)* |
| Less than 45 | 1282 | 12 (9.3) | 0.07 (0.02, 1.20) |
| 45 – 54 | 525 | 21 (21.0) | 0.48 (0.19, 1.22) |
| 55 – 64 | 470 | 15 (16.7) | 0.40 (0.11, 1.51) |
| 65 and older | 277 | 23 (53.0) | reference |
| Age (continuous) (mean/SD) | 45.2 (15.5) | 1.06 (1.04, 1.08) | |
| Gender | |||
| Male | 862 | 25 (56.7) | 1.55 (0.67, 3.56) |
| Female | 1692 | 46 (43.4) | reference |
| Employment status | |||
| Employed | 1251 | 24 (44.8) | 0.88 (0.34, 2.13) |
| Unemployed | 1303 | 47 (55.2) | reference |
| Poverty (2014 guidelines) | |||
| Below the poverty | 1688 | 43 (45.4) | 0.26 (0.11, 0.64) |
| Above the poverty | 296 | 16 (54.6) | reference |
| Country of Birth | |||
| Mexico | 1648 | 37 (46.7) | reference |
| United States | 866 | 32 (53.3) | 1.78 (0.77, 4.14) |
| Medical Status | |||
| Diabetes (ADA 2010 definition) | 728 | 29 (66.8) | 2.62 (1.19, 5.79) |
| Insulin Resistance (HOMA>3.15) | 910 | 23 (47.6) | 0.92 (0.45, 1.90) |
| Body Mass Index | |||
| BMI<25 | 419 | 10 (16.9) | reference |
| 25≤BMI<30 | 819 | 25 (33.0) | 0.91 (0.23, 3.65) |
| 30≤BMI<35 | 741 | 18 (25.9) | 0.82 (0.18, 3.87) |
| 35≤BMI<40 | 337 | 11 (21.1) | 1.60 (0.31, 8.17) |
| BMI ≥ 40 | 223 | 6 (3.1) | 0.31 (0.06, 1.49) |
| Obese | |||
| Yes | 1297 | 35 (49.6) | 0.96 (0.40, 2.32) |
| No | 1245 | 36 (50.4) | reference |
| Metabolic Syndrome | |||
| Yes | 1172 | 39 (59.2) | 1.52 (0.61, 3.75) |
| No | 1204 | 30 (40.7) | reference |
| Smoking Status | |||
| Never | 1390 | 32 (19.0) | reference |
| Former | 758 | 31 (65.5) | 2.09 (1.01, 4.30) |
| Current | 406 | 8 (15.5) | 1.43 (0.34, 5.71) |
| Family history of cancer | |||
| Any relative | 968 | 34 (40.7) | 1.09 (0.47, 2.53) |
| Father | 304 | 14 (17.8) | 1.67 (0.48, 5.84) |
| Mother | 316 | 6 (5.9) | 0.44 (0.16, 1.25) |
| At least one sibling | 249 | 14 (25.4) | 1.31 (0.46, 3.69) |
| Other male relative | 226 | 7 (11.1) | 0.55 (0.17, 1.75) |
| Other female relative | 328 | 12 (16.0) | 0.61 (0.22, 1.73) |
Unweighted frequency, weighted percentages and OR (95%CI)
Table 2 shows the relationship of characteristics of the participants to family histories of cancer in relatives. Participants who met the definition for metabolic syndrome had an odds ratio of 1.73 (95%CI 1.26, 2.37) of having had at least one parent with cancer, and an odds ratio of 3.40(95%CI 1.06, 10.91) if two or more siblings also had cancer.
Table 2. Participant-reported family history of cancer of the participants and association with selected risk factors for cancer among the participants themselves (n=2,547).
| Family history of cancer | Characteristics of the CCHC participant | |||
|---|---|---|---|---|
| Diabetes | Obesity | Insulin Resistance | Metabolic Syndrome (41) | |
| OR* (95% C.I.) | OR* (95% C.I.) | OR* (95% C.I.) | OR* (95% C.I.) | |
| At least 1 parent | 1.14 (0.79, 1.64) | 1.13 (0.82, 1.56) | 1.09 (0.78, 1.51) | 1.73 (1.26, 2.37) |
| Father | 0.94 (0.58, 1.51) | 1.19 (0.77, 1.83) | 1.44 (0.97, 2.13) | 1.36 (0.90, 2.06) |
| Mother | 1.34 (0.87, 2.05) | 1.11 (0.74, 1.66) | 0.86 (0.57, 1.31) | 1.89 (1.28, 2.77) |
| Both parents | 1.13 (0.47, 2.68) | 1.55 (0.68, 3.54) | 1.30 (0.52 – 3.28) | 1.17 (0.53, 2.62) |
| At least 1 sibling | 1.17 (0.46, 2.97) | 1.78 (0.68, 4.61) | 0.74 (0.28, 1.93) | 1.29 (0.46, 3.61) |
| 2 or more siblings | 0.64 (0.19, 2.17) | 2.68 (0.81, 8.93) | 0.80 (0.22, 2.97) | 3.40 (1.06, 10.91) |
| Any relative* | 1.14 (0.83, 1.55) | 1.22 (0.92, 1.62) | 1.01 (0.76, 1.34) | 1.45 (1.12, 1.88) |
Weighted Odds Ratio and 95% Confidence Interval
In order to determine independent, significant variables we built a weighted multivariable logistic regression model including variables we found to have a significant association with cancer together with gender, cancer in parent, smoking status and country of birth. We constructed two-way statistical interactions to examine for possible effect modifications in the model and found that the interaction between age and diabetes status was statistically significant (p=0.0078). After including the interaction effect in the model the adjusted odds ratio for cancer in diabetes versus no diabetes was 22.98, (95%CI 2.84, 185.76) with change of 0.96 (95% CI 0.93, 0.99) for each year increase in age. Finally we determined that the effect of age on the diabetes risk was bimodal; those under the age of 70 with diabetes were more likely to report cancer (OR 3.57, 95% CI 1.32, 9.62) and those over 70 less likely if they had diabetes (OR 0.64 95% CI 0.16, 2.58). We were unable to determine independently whether diabetes was significantly associated with any single cancer site due to small numbers (data not shown).
Family history of cancer among first degree relatives (parents)
A higher percentage of the parents compared to participants were born in Mexico (80.8% of mothers and 77.2% of fathers). Among parents participant-reported diabetes was also high (34.4% in mothers and 24.9% in fathers). Diabetes was reported for 13.9% of mothers and 12.7% of fathers. Diabetes status was missing for 104 mothers and 304 fathers as was cancer status of 61 mothers and 248 fathers. Table 3 lists in order of frequency the most commonly reported cancers among parents and association with diabetes in the parent. Being born in the U.S. was significant for all cancers (overall Odds Ratio of 1.38, 95% CI 1.12,1.70, for both genders). Regardless of gender and excluding gender-specific cancer sites, lung cancer was by far the most frequent with 100 reports (2.1%). The next three most frequently reported (excluding gender-specific sites) were all cancers of the gastrointestinal system: stomach (46; 0.95%), liver (36; 0.74%) and colon/rectum (24; 0.43%). Gender-specific cancer sites were also frequently reported with cervix/uterus leading with 66 reports in mothers (2.6%) followed by breast (2.4%) and ovarian (1.4%) cancers. In fathers prostate cancer was second most common cancer (2.6%) after lung cancer (3.1%).
Table 3.
Risk factors associated with reported cancer in parents.
| Cancer sites | Reported disease in parent | Odds Ratio for risk factors for cancer in parents | |||
|---|---|---|---|---|---|
| Cancer | Diabetes | Diabetes | Born in U.S. | Born in Mexico | |
| n (%) | OR (95% CI) | ||||
| Both parents n=4,827 | |||||
| All cancers | 623 (12.07%) | 159 (25.52%) | 0.82 (0.68, 0.99) | 1.38 (1.12, 1.70) | 0.73 (0.60, 0.89) |
| Lung | 100 (2.07%) | 21 (21.00%) | 0.69 (0.42, 1.13) | 1.37 (0.85, 2.22) | 0.86 (0.54, 1.38) |
| Stomach | 46 (0.95%) | 11 (23.91%) | 0.82 (0.41, 1.62) | 1.92 (1.01, 3.67) | 0.59 (0.31, 1.11) |
| Liver | 36 (0.74%) | 9 (25.00%) | 0.87 (0.41, 1.86) | 0.97 (0.41, 2.36) | 1.07 (0.46, 2.46) |
| Colon/Rectum | 24 (0.43%) | 9 (37.50%) | 1.57 (0.68, 3.59) | 2.93 (1.28, 6.72) | 0.30 (0.14, 0.68) |
| Mother n=2,513 | |||||
| All Cancers | 318 (12.32%) | 108 (33.96%) | 1.02 (0.79, 1.30) | 1.24 (0.92, 1.67) | 0.79 (0.59, 1.05) |
| Cervical/Uterine | 66 (2.62%) | 17 (25.76%) | 0.71 (0.40, 1.24) | 1.03 (0.53, 1.98) | 0.94 (0.51, 1.75) |
| Breast | 60 (2.39%) | 19 (31.67%) | 0.95 (0.55, 1.65) | 1.13 (0.58, 2.20) | 0.93 (0.49, 1.76) |
| Ovarian | 34 (1.35%) | 10 (29.41%) | 0.85 (0.40, 1.79) | 1.93 (0.89, 4.22) | 0.48 (0.23, 1.00) |
| Lung | 27 (1.07%) | 10 (34.04%) | 1.2 (0.54, 2.64) | 1.18 (0.44, 3.14) | 0.81 (0.32, 2.02) |
| Stomach | 22 (0.87%) | 5 (22.73%) | 0.6 (0.22, 1.64) | 1.1 (0.37, 3.27) | 1.04 (0.35, 3.10) |
| Father n=2,314 | |||||
| All Cancers | 305 (13.9%) | 51 (16.72%) | 0.6 (0.44, 0.83) | 1.54 (1.16, 2.05) | 0.68 (0.52, 0.89) |
| Lung | 73 (3.1%) | 11 (15.07%) | 0.61 (0.31, 1.16) | 1.42 (0.82, 2.47) | 0.94 (0.54, 1.64) |
| Prostate | 60 (2.57% | 9 (15.00%) | 0.61 (0.30, 1.24) | 0.93 (0.47, 1.86) | 1.03 (0.56, 1.93) |
| Liver | 25 (1.09%) | 3 (12.00%) | 0.47 (0.14, 1.57) | 0.89 (0.30, 2.61) | 1.14 (0.42, 3.07) |
| Stomach | 24 (1.04%) | 6 (25.00%) | 1.15 (0.45, 2.91) | 2.81 (1.22, 6.47) | 0.4 (0.17, 0.90) |
Comparison with different populations
Table 4 compares ranked cancer frequency for the parents with cancer registry data from other populations in the U.S. and Mexico. In most of these populations the two most frequent cancers in women are cervix/uterus and breast. However, liver and stomach cancers were ranked higher in the mothers of our participants than in any other U.S. population. These rankings resembled more closely those from Mexico. Similar analysis for fathers shows that the two leading reported cancer sites, lung and prostate cancer, are also among the top three leading cancer sites for Hispanic men in the U.S and in Mexico, as well as for non-Hispanic men across the U.S.. However, the next two cancer sites, liver and stomach, had higher rankings in fathers of our participants than in any other population of in the U.S., and again resembled the ranking in Mexico.
Table 4.
| WOMEN | CCHC Data Mothers (n=2,513) | Cancer Registries Data | |||
|---|---|---|---|---|---|
| RANK | Hispanic women in Texas(28) | Women in Mexico(30) | Hispanic women in U.S.(29) | Non-Hispanic women in U.S.(29) | |
| 1 | Cervix/Uteri* | Breast | Breast | Breast | Breast |
| 2 | Breast | Colorectal | Cervix | Colorectal | Lung |
| 3 | Ovary | Lung | Stomach | Lung | Colorectal |
| 4 | Lung | Uterus | Liver | Uterus | Uterus |
| 5 | Stomach | Thyroid | Colorectal | Thyroid | Thyroid |
| 6 | Liver | Lymphoma | Ovary | Lymphoma | Melanoma |
| 7 | Colorectal | Kidney | Uterus | Ovary | Lymphoma |
| 8 | Bladder | Cervix | Lung | Cervix | Ovary |
| 9 | Leukemia | Ovary | Gallbladder | Kidney | Kidney |
| 10 | Kidney | Pancreas | Leukemia | Pancreas | Pancreas |
| 11 | Pancreas | Stomach | Pancreas | Leukemia | Leukemia |
| MEN | CCHC Data Fathers (n=2,314) | Cancer Registries Data | |||
| RANK | Hispanic men in Texas(28) | Men in Mexico(30) | Hispanic men in U.S.(29) | Non-Hispanic men in U.S.(29) | |
| 1 | Lung | Prostate | Prostate | Prostate | Prostate |
| 2 | Prostate | Colorectal | Lung | Colorectal | Lung |
| 3 | Liver | Lung | Stomach | Lung | Colorectal |
| 4 | Stomach | Kidney | Colorectal | Kidney | Bladder |
| 5 | Head & Neck | Liver | Liver | Lymphoma | Melanoma |
| 6 | Colorectal | Lymphoma | Leukemia | Bladder | Lymphoma |
| 7 | Bone | Bladder | Lymphoma | Liver | Kidney |
| 8 | Leukemia | Stomach | Kidney | Stomach | Mouth/pharynx |
| 9 | Throat | Pancreas | Pancreas | Leukemia | Leukemia |
| 10 | Pancreas | Leukemia | Testicular | Pancreas | Pancreas |
| 11 | Esophagus | Mouth/Pharynx | Bladder | Mouth/Pharynx | Liver |
CCHC Participants were not able to distinguish between cervical and uterine cancers
… Insufficient cases to derive reliable estimates
Comparison with local cancer registry data
Comparing our rankings with age-adjusted incidence of clinically-diagnosed cancers from the Cameron County Cancer Registry (2005-2009) confirms our key observations, particularly the higher frequency of gastrointestinal system cancers than in other populations.(28) Table 5 shows strikingly higher incidence in Cameron County of liver and stomach cancer among Hispanic men compared with non-Hispanic men, but lower age-adjusted incidence of the more common cancers such as the lung, bronchus and colon. In both men and women pancreatic cancer incidence is higher in Hispanics.
Table 5.
Texas Cancer Registry age adjusted incidence of cancers in Cameron County, Texas, comparing rates in Hispanic and non-Hispanic men and women.(28)
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Age adjusted incidence 2005-9 | Total | Hispanic | Non-Hispanic whites | Total | Hispanic | Non-Hispanic whites |
| All invasive cancers | 319. 0 | 334.8 | 315.7 | 484.0 | 486.5 | 527.0 |
| Gastrointestinal System | ||||||
| Stomach | 7.1 | 9.6 | … | 12.8 | 16.4 | 7.6 |
| Liver | 5.8 | 8.1 | … | 17.8 | 24.5 | 9.0 |
| pancreas | 8.6 | 12.1 | 6.7 | 10.6 | 14.3 | 11.8 |
| Colon excluding rectum | 28.0 | 23.6 | 19.4 | 37.5 | 35.3 | 40.8 |
| Other common sites | ||||||
| Lung & bronchus | 25.2 | 19.4 | 40.0 | 63.9 | 62.7 | 77.0 |
| Kidney & Renal Pelvis | 11.4 | 12.8 | 7.5 | 21.7 | 22.9 | 23.0 |
| Leukemia | 7.8 | 7.6 | 5.0 | 15.4 | 16.1 | 15.2 |
| Gender specific sites | ||||||
| Breast | 87.7 | 88.3 | 79.5 | |||
| Corpus uteri | 17.0 | 17.4 | 13.7 | |||
| Cervix uteri | 12.6 | 13.5 | … | |||
| Ovary | 12.3 | 10.9 | 12.3 | |||
| Prostate | 126.4 | 127.0 | 137.0 | |||
… Insufficient cases to derive reliable estimates
Conclusions
We show that gastrointestinal cancers, particularly liver cancer, are more frequent in the Mexican American population than other ethnic groups in the U.S. We also show that the elevated frequency of these cancers tracks with health-disparity risk factors: obesity, diabetes and metabolic syndrome. We also demonstrate that the diabetes epidemic may underlie increases in gastrointestinal cancers particularly in people under the age of 70 years. These data suggest that gastrointestinal system cancers will be important components in the growing pandemic of NCDs both in the U.S. and LMIC regions globally.(2)
Sixty percent of all cancers globally are in LMICs where there is often lack of access to cancer diagnosis and treatment; nearly a third of cases being in people under the age of 60 years.(2) Similar conditions to those in LMICs are found on the US/Mexico border.(25) The predictions of a percentage increase in cancer incidence of 58% in LMICs by 2030 compares with 40% in high income countries.(1) Liver cancer is among the most deadly cancers with a world incidence for 2012 of 15.3/100,000, but mortality of 14/100,000.(2) Among Hispanics it has half the five-year cancer-specific survival rate of non-Hispanic whites in both sexes, accounting for 11% of cancer deaths in Hispanics nationally. This is possibly due to late diagnosis and inadequate access to health care, but also to obesity.(3,13)
The community we study is in the poorest county in the U.S. However, the local cancer registry reports incidence and distinguishes ethnicity, at least for those with health insurance, but we are informed that only hospitals report to this registry (personal communication B. Smith). Seventy percent of the cohort have no insurance, and will not reach these hospitals. However, since the size and observation period of the cohort were too small to obtain accurate incidence data, we accessed registry data to validate our ranking of cancer sites. The high incidence of liver cancer in Hispanics in the registry (third most common among men, and sixth among women in our study) was striking. This contrasts with national data where liver cancer ranks below 13th.(31) For men this local ranking is considerably higher than among Hispanic and non-Hispanic males nationally and for women nationally liver cancer is not even among the top 10 most common cancers. We have already reported high rates of elevated alanine transaminase and genetic factors for liver disease in our population as well as high frequency of end-stage liver disease, and more recently, high rates of fatty liver on ultrasound.(32-36) Alcohol abuse is not highly prevalent and hepatitis virus infections are not common.(34) Together these observations suggest there may be high frequency of non-alcoholic fatty liver disease. Indeed, local physicians report seeing large numbers of patients with nonalcoholic steatohepatitis (NASH) which is likely a driver of liver cancer in this population.
The greater odds of having any cancer in participants with diabetes, particularly in those under the age of 70 years, suggests that diabetes mostly impacts occurrence o early cancers. Furthermore a participant with the metabolic syndrome had a higher likelihood of reporting a family history of cancer suggesting that the components of the metabolic syndrome, particularly obesity and diabetes, contribute to clustering of cancers in the immediate family. (37,38)
A strength of our approach is the use of data from relatives of the CCHC participants which increased the sample size by as much as 10-fold and expanded the timeline over many years. Nevertheless, there are considerable limitations to our study. Use of nested data from our cohort does not account for the possibility that not all the relatives are members of the target community and the timeframe is unclear. Furthermore, the reliability of the reports of cancer, particularly common metastatic sites, cannot be confirmed. The numbers of cohort participants themselves with cancer were too small to analyze for individual cancer sites, and we do not have actual mortality data or independent cancer diagnosis even for the participants. Data from relatives were limited and did not include age at cancer onset or at death, and could not be validated with pathological diagnoses. Among relatives the data from parents were the most complete and we have a reliable denominator. Consequently we used only data from parents in most of our analyses among relatives. Regardless of these important limitations, this approach using participant-reported data produces the only comprehensive information we have on cancer in this community. To generate accurate data prospectively would require considerable expenditure of time and money to obtain a sufficiently large sample size, number of end points and longer follow-up period for statistical power. However, the self-reported approach we used has been validated in one study by correlating up to 84% of self-reported cancers with pathology from medical records.(39) Given these limitations and to validate our data further, we used local registry data to confirm the relative importance of gastrointestinal cancers in local Hispanic patients.(table 5) Local registry data, however, also suffers from limitations since the most consistent reporting is from local hospitals and uninsured patients such as those in our cohort receive hospital care from other centers in Texas funded to take them, or in Mexico, from where we have no data.(11,24) How the registry data may be skewed by winter visitors (Winter Texans) is illustrated by the high ranking of melanoma in Texas cancer registry data (13.7% overall, but only 3.8% in Hispanics).(28) Finally, we could not compare our data directly with registry data because we were unable to estimate age-adjusted incidence. Despite these limitations the prominence of gastrointestinal cancers is consistent and striking.
The Mexican-American population on the U.S./Mexico border has limited access to health care and preventive practices such as screening for cancer.(40) Obesity and diabetes rates in this community are among the highest reported in the nation. These data show the importance of gastrointestinal cancers, particularly liver cancer, in such disadvantaged populations. Both nationally and globally the only practical approach is prevention. These data are therefore useful for planning cancer prevention programs. Cohorts such as ours also provide a useful testing ground for low cost, low-tech screening, and use of new biomarkers to detect early disease. Finally, the high prevalence of obesity in this population is most likely due to lifestyle behaviors such as inadequate physical activity and poor eating habits which also highlights the need for obesity prevention and behavioral change interventions.
Acknowledgments
We thank Dr. Brian Smith, director of Region 11, Texas Department of State Health Services, for reviewing the manuscripts and helpful suggestions. We thank our cohort recruitment team, particularly Rocio Uribe and Julie Ramirez-Gomez. We also thank Marcela Montemayor and other laboratory staff for their contribution, Gloria Sanchez and Pablo Sanchez for our database management and Christina Villarreal for administrative support. We thank Valley Baptist Medical Center, Brownsville for providing us space for our Center for Clinical and Translational Science Clinical Research Unit. We finally thank the community of Brownsville and the participants who so willingly participated in this study in their city.
Grant Support-: This work was supported by MD000170 P20 funded from the National Institute on Minority Health and Health disparities (NIMHD), and the Centers for Clinical and Translational Science Award 1U54RR023417-01 from the National Center for Research Resources (NCRR).
Funding sources: This work was supported by MD000170 P20 funded from the National Center on Minority Health and Health disparities (NCMHD), and the Centers for Clinical and Translational Science Award UL1 TR000371 from the National Center for Research Resources (NCRR).
Footnotes
Ms Garza and Ms Vatcheva declare they have no conflict of interest. Drs. Fisher-Hoch, Rahbar, Fallon, Pan, and McCormick declare they have no conflict of interest.
Compliance with Ethical Standards: Informed Consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study. No identifying information was used in this article. This study and all procedures including written informed consent forms were approved by the Committee for the Protection of Human Subjects at UTHealth, Houston, approval numbers HSC-SPH-03-007 A and B. The study was explained to all participants in their language of choice (Spanish or English) before obtaining written consent.
No animals were used in this study.
References
- 1.World Health Organisation. Global Status Report on non-communicable diseases. Italy: World Health Organisation; Alwan A. 1-176. 4-1-2011. [Google Scholar]
- 2.International Agency for Research on Cancer. World Cancer Report 2014. Lyon/London: World Health Organisation; 2014. [Google Scholar]
- 3.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Yeh HC, Platz EA, Wang NY, Visvanathan K, Helzlsouer KJ, Brancati FL. A prospective study of the associations between treated diabetes and cancer outcomes. Diabetes Care. 2012;35:113–118. doi: 10.2337/dc11-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Census Bureau. Hispanic America by the Numbers. [article online] 2011 Available from http://www.infoplease.com/spot/hhmcensus1.html.
- 6.Kanna B, Fersobe S, Soni A, Michelen W. Leading Health Risks, Diseases and Causes Of Mortality Among Hispanics in the United States of America. The Internet Journal of Health. 2008;8 doi: 10.5580/273c. [DOI] [Google Scholar]
- 7.Pinheiro PS, Sherman RL, Trapido EJ, Fleming LE, Huang Y, Gomez-Marin O, Lee D. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162–2169. doi: 10.1158/1055-9965.EPI-09-0329. [DOI] [PubMed] [Google Scholar]
- 8.Balluz LS, Okoro CA, Mokdad A. Association between selected unhealthy lifestyle factors, body mass index, and chronic health conditions among individuals 50 years of age or older, by race/ethnicity. Ethn Dis. 2008;18:450–457. [PubMed] [Google Scholar]
- 9.Ennis SR, Rios-Vargas M, Albert NG. 2010 Census Briefs. United States Census Bureau; The Hispanic Population:2010. C201088 04, 1-16. 2011. [Google Scholar]
- 10.United States Census Bureau. USA QuickFacts. U.S. Department of Commerce; 2012. [Google Scholar]
- 11.Fisher-Hoch SP, Vatcheva KP, Laing ST, Hossain MM, Rahbar MH, Hanis CL, Brown HS, III, Rentfro AR, Reininger BM, McCormick JB. Missed opportunities for diagnosis and treatment of diabetes, hypertension, and hypercholesterolemia in a Mexican American population, Cameron County Hispanic Cohort, 2003-2008. Prev Chronic Dis. 2012;9:E135. doi: 10.5888/pcd9.110298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012;62:283–298. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 13.American Cancer Society. Cancer facts and figures for Hispanics/Latinos 2012- 2014. Atlanta, Ga: American Cancer Society; 2014. 1-7-2014. [Google Scholar]
- 14.Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund E. The validity of race and Hispanic origin reporting on death certificates in the United States. Vital Health Stat 2. 2008:1–23. [PubMed] [Google Scholar]
- 15.Murphy SL, Xu Jiaquan, Kochanek KD. National Vital Statistics Reports. 4. Vol. 60. National Center for Vital Statistics; Death: Preliminary Data for 2010; pp. 1–52. 1-11-2012. [Google Scholar]
- 16.Rosenberg HM, Maurer JD, Sorlie PD, Johnson NJ, MacDorman MF, Hoyert DL, Spitler JF, Scott C. Quality of death rates by race and Hispanic origin: a summary of current research, 1999. Vital Health Stat 2. 1999:1–13. [PubMed] [Google Scholar]
- 17.Alexandraki I. The United States-Mexico border: an area in need of cancer screening interventions. J Womens Health (Larchmt) 2011;20:653–655. doi: 10.1089/jwh.2010.2700. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. Heath, United States, 2013: with Special Feature on Prescription Drugs. 2015. 1-12-2015. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Heath, United States, 2013. 2015 [Google Scholar]
- 20.Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital Health Stat 10. 2009:1–157. [PubMed] [Google Scholar]
- 21.Benabe JE, Rios EV. Kidney disease in the Hispanic population: facing the growing challenge. J Natl Med Assoc. 2004;96:789–798. [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher-Hoch SP, Vatcheva KP, Laing ST, Hossain MM, Rahbar MH, Hanis CL, Brown HS, III, Rentfro AR, Reininger BM, McCormick JB. Missed opportunities for diagnosis and treatment of diabetes, hypertension, and hypercholesterolemia in a mexican american population, cameron county Hispanic cohort, 2003-2008. Prev Chronic Dis. 2012;9:E135. doi: 10.5888/pcd9.110298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez AG, Weiss NS, Holden AE, Suarez L, Cooper SP, Munoz E, Naylor SL. Incidence and risk factors for hepatocellular carcinoma in Texas Latinos: implications for prevention research. PLoS One. 2012;7:e35573. doi: 10.1371/journal.pone.0035573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reininger BM, Barroso CS, Mitchell-Bennett L, Chavez M, Fernandez ME, Cantu E, Smith KL, Fisher-Hoch SP. Socio-ecological Influences on Health-Care Access and Navigation Among Persons of Mexican Descent Living on the U.S./Mexico Border. J Immigr Minor Health. 2012 doi: 10.1007/s10903-012-9714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher-Hoch SP, Rentfro AR, Salinas JJ, Perez A, Brown HS, Reininger BM, Restrepo BI, Wilson JG, Hossain MM, Rahbar MH, Hanis CM, McCormick JB. Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004-2007. Prev Chronic Dis. 2010;7:A53. [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Institute and Centers for Disease Control and Prevention. State Cancer Profiles. 2012 [Google Scholar]
- 28.Texas Cancer Registry. Age-Adjusted Invasvive Cancer Incidence in Texas. 2012 10-29-2012. [Google Scholar]
- 29.Centers for Disease Control and Prevention. United States Cancer Statistics. CDC; 2012. Public Information Data. 10-29-2012. [Google Scholar]
- 30.IARC. Globocan 2008 Fast Stats. Lyon, France: World Health Organization; 2012. 10-29-2012. [Google Scholar]
- 31.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA, Edwards BK. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Nat Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Qu HQ, Rentfro AR, Grove ML, Mirza S, Lu Y, Hanis CL, Fallon MB, Boerwinkle E, Fisher-Hoch SP, McCormick JB. PNPLA3 Polymorphisms and Liver Aminotransferase Levels in a Mexican American Population. Clin Invest Med. 2012;35:E237. doi: 10.25011/cim.v35i4.17153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu HQ, Li Q, Grove ML, Lu Y, Pan JJ, Rentfro AR, Bickel PE, Fallon MB, Hanis CL, Boerwinkle E, McCormick JB, Fisher-Hoch SP. Population-based Risk Factors for Elevated Alanine Aminotransferase in a South Texas Mexican-American Population. Arch Med Res. 2012 doi: 10.1016/j.arcmed.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan JJ, Qu HQ, Rentfro A, McCormick JB, Fisher-Hoch SP, Fallon MB. Prevalence of metabolic syndrome and risks of abnormal serum alanine aminotransferase in Hispanics: a population-based study. PLoS One. 2011;6:e21515. doi: 10.1371/journal.pone.0021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez A, Anzaldua M, McCormick J, Fisher-Hoch SP. High frequency of chronic end-stage liver disease and hepatocellular carcinoma in a Hispanic population. J Gastroenterol Hepatol. 2004;19:289–295. doi: 10.1111/j.1440-1746.2003.03277.x. [DOI] [PubMed] [Google Scholar]
- 36.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 37.Hanis CL, Chu HH, Lawson K, Hewett-Emmett D, Barton SA, Schull WJ, Garcia CA. Mortality of Mexican Americans with NIDDM. Retinopathy and other predictors in Starr County, Texas. Diabetes Care. 1993;16:82–89. doi: 10.2337/diacare.16.1.82. [DOI] [PubMed] [Google Scholar]
- 38.Guillaume M, Lapidus L, Beckers F, Lambert A, Bjorntorp P. Familial trends of obesity through three generations: the Belgian-Luxembourg child study. Int J Obes Relat Metab Disord. 1995;19(3):S5–S9. [PubMed] [Google Scholar]
- 39.Airewele G, Adatto P, Cunningham J, Mastromarino C, Spencer C, Sharp M, Sigurdson A, Bondy M. Family history of cancer in patients with glioma: a validation study of accuracy. J Natl Cancer Inst. 1998;90:543–544. doi: 10.1093/jnci/90.7.543. [DOI] [PubMed] [Google Scholar]
- 40.Texas Department of State Health Services. Behavioral Risk Factor Surveillance System. 2012 10-29-2012. [Google Scholar]
- 41.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]