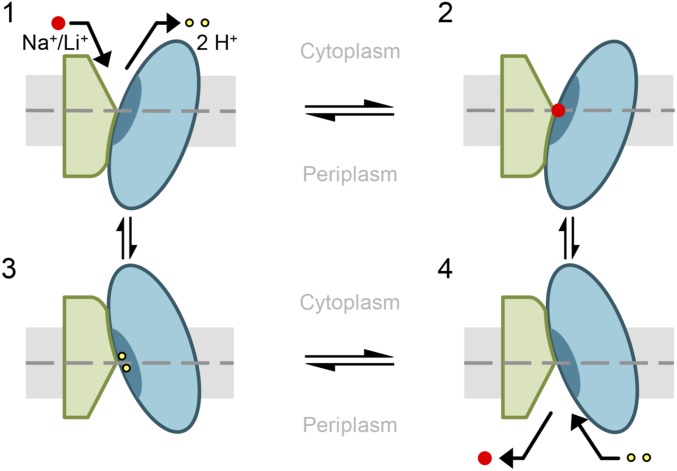
Fig. 5.
Proposed alternating-access mechanism of NhaA. Schematic representation of NhaA viewed parallel to the membrane. The two domains are shown in green (interface) and blue (core). TM V including the binding site is indicated as a dark blue segment in the core domain. The membrane is shown in gray, and a dashed line indicates its center. NhaA in the “cytoplasmic open” conformation releases two protons (yellow dots) (1) and binds Na+/Li+ (red dots) (2). After Na+/Li+ binding, the core domain translocates by a rotational upward translation, closing the cytoplasmic and opening the periplasmic funnel (3). Thereby the Li+/Na+ is released into the periplasm and two protons can bind (4). Due to the proton binding, NhaA switches back into the cytoplasmic open conformation and a new transport cycle starts (1). TM V including the binding site remains immobile relative to the membrane throughout the entire cation binding and translocation process.