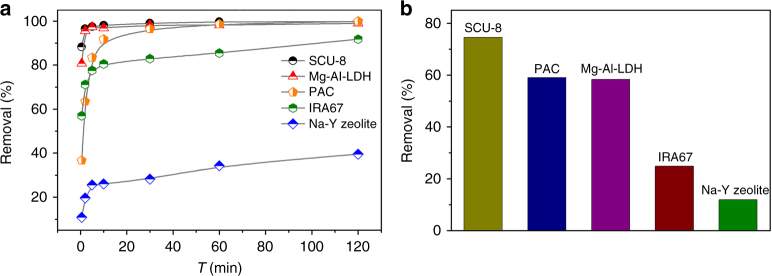
Fig. 4.
The results of PFOS sorption kinetics and selectivity by various materials. a A comparison of PFOS sorption kinetics by SCU-8, Mg-Al-LDHs, IRA67, PAC, and Na-Y zeolite, with the same solid-to-liquid ratio of 0.2 mmol/40 ml and the initial PFOS concentration of 1 mg l−1. b A comparison of PFOS removal percentages at the equilibrium state in the presence of large excess of multiple competing anions Cl−, NO3 −, SO4 2−, and CO3 2− by SCU-8, Mg-Al-LDHs, IRA67, PAC, and Na-Y zeolite. The concentration of each competing anion is 50 mg l−1 and the initial PFOS concentration of 1 mg l−1