Abstract
The risk of acute and chronic kidney disease remains higher in HIV-infected persons than in the general population, and kidney disease in HIV-infected persons is associated with poor outcomes, including increased mortality. HIV-associated nephropathy occurs less frequently in the era of antiretroviral therapy. HIV immune complex kidney disease is being diagnosed more frequently, but the term is currently used to refer to a heterogeneous group of kidney diseases. Comorbid chronic kidney disease poses a growing burden in HIV-infected persons due to an overrepresentation of risk factors such as black race, diabetes, hypertension, and coinfection with hepatitis C virus. Drug-induced kidney toxicity also remains a concern. This article summarizes a presentation by Christina M. Wyatt, MD, at the Ryan White HIV/AIDS Program Clinical Care Conference held in New Orleans, Louisiana, in December 2015.
Keywords: HIV, acute kidney injury, comorbid kidney disease, chronic kidney disease, diabetes, tenofovir, hepatitis C virus
Kidney disease in HIV-infected persons manifests in a variety of ways, including acute kidney injury (AKI), HIV-associated kidney disease, comorbid chronic kidney disease (CKD), and treatment-related kidney toxicity. The burden of CKD and end-stage renal disease (ESRD) remains high in the HIV-infected population.
There are several important caveats to consider when diagnosing and managing kidney disease in HIV-infected persons. Glomerular filtration rate (GFR) estimates are not well validated in this population. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, which incorporates serum creatinine level and demographic factors, appears to provide the most accurate estimates among HIV-infected persons who are stable on antiretroviral therapy. However, there are strengths and limitations for all currently used equations, and creatinine clearance by Cockcroft-Gault calculation remains the recommended kidney function estimate for drug dosing. Several antiretroviral and other medications (eg, dolutegravir, rilpivirine, trimethoprim, and the pharmacoenhancers cobicistat and ritonavir) can interfere with creatinine secretion without affecting true GFR. In addition, creatine supplements and diets high in animal protein can increase levels of serum creatinine, resulting in an inaccurate estimate of GFR. Cystatin C testing may be helpful in such situations but should be used with caution in patients with HIV infection. Although normal cystatin C test results can be reassuring, abnormal cystatin C test results could reflect a decrease in GFR or an increase in systemic inflammation.1–3
Acute Kidney Injury
AKI is more common in HIV-infected persons than in the general population and is associated with increased risk of adverse outcomes such as heart failure, cardiovascular disease, ESRD, and death.4 The incidence of AKI has decreased since the introduction of potent antiretroviral therapy but remains substantial,5 with evidence in more recent years of an increase in incidence of more severe AKI.6 Data from a national sample of hospital admissions showed that an increasing proportion of hospitalizations among HIV-infected persons were complicated by dialysis-dependent AKI (Figure 1).6 Although these data could reflect more frequent use of dialysis, the association between severe AKI and increased risk of mortality was also stronger in later years of the study period.
Figure 1.
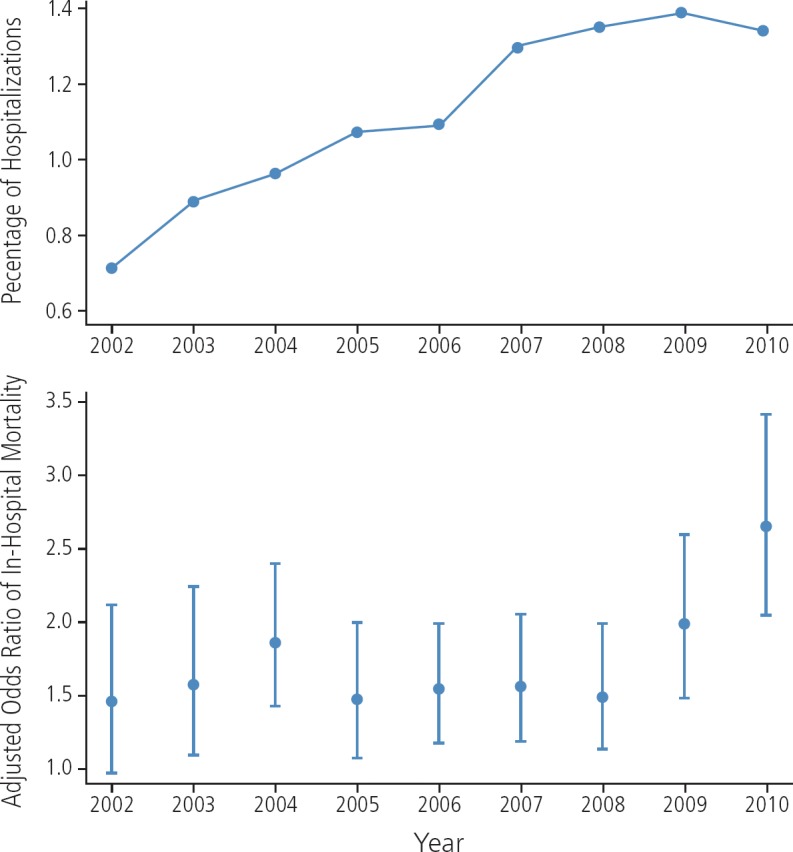
Proportion of hospitalizations among HIV-infected persons complicated by dialysis-dependent acute kidney injury (top), and adjusted odds ratio of in-hospital mortality associated with dialysis-dependent acute kidney injury (bottom). Adapted from Nadkarni et al.6
Detailed data on the etiology of AKI in the context of HIV infection are outdated. In a 2005 study, the most common causes of AKI were systemic infections, including AIDS-defining infections.7 More recent data from the National Inpatient Sample mentioned above indicate that sepsis remains a common risk factor for severe AKI, along with other traditional risk factors (eg, diabetes, hypertension, CKD, and liver disease) in the aging HIV-infected population.6
HIV-Associated Kidney Disease
HIV-associated nephropathy (HIVAN) was the first kidney disease described in HIV-infected persons but is now infrequently encountered in populations with access to antiretroviral therapy. It is most commonly seen in persons who are newly diagnosed with late-stage HIV infection or in those who have discontinued antiretroviral therapy, and it may present as AKI or CKD. In addition to HIVAN, the spectrum of HIV-associated kidney disease includes HIV immune complex kidney disease (HIVICK) and, less commonly, thrombotic microangiopathy.
HIVAN is classically associated with rapid progression to ESRD, occurs in advanced HIV disease, and is observed almost exclusively in persons of African descent, who account for approximately 90% of HIVAN-related cases of ESRD. HIVAN has a distinct histology, representing a collapsing form of focal segmental glomerulosclerosis (FSGS). The pathogenesis of HIVAN requires local HIV infection of the kidney, with the virus infecting tubular and glomerular epithelial cells. Along with local infection of the kidney, systemic HIV infection and systemic immune dysfunction may also contribute to disease pathogenesis. The strong racial disparity in HIVAN and associated ESRD is related to polymorphisms in the APOL1 gene, a gene on chromosome 22 that encodes apolipoprotein 1 and is associated with susceptibility to trypanosomiasis. Antiretroviral therapy is the recommended initial treatment for HIVAN, and there is some evidence of benefit with adjunctive therapies including angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and corticosteroids. Most experts would limit the use of corticosteroids to patients with progressive disease despite antiretroviral therapy.8
HIVICK has not been as well studied, in part because the term has been used to refer to a broad spectrum of heterogeneous glomerular diseases. The causal relationship between HIV infection and HIVICK is less clear than for HIVAN, and whether antiretroviral therapy can reverse or delay the progression of HIVICK is also unclear.
Comorbid Kidney Disease
Although there has been a decrease in the incidence of HIVAN, comorbid CKD is a growing burden in the HIV-infected population. Traditional risk factors for CKD, including black race, diabetes, hypertension, and coinfection with HCV, are overrepresented in this population, making it difficult to distinguish the contribution of HIV infection. In a study by Medapalli and colleagues, HIV infection and diabetes had an additive effect on risk for CKD progression (Figure 2).9 Data from animal studies suggest that this may be related to synergistic inflammatory pathways upregulated by diabetes and HIV infection.10
Figure 2.
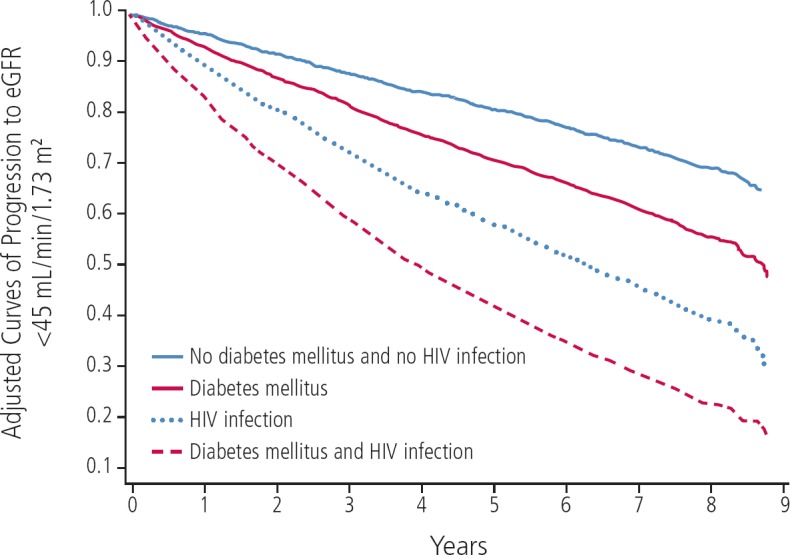
Additive effect of HIV infection and diabetes on progression of chronic kidney disease. Adapted from Medapalli et al.9
HCV infection is also associated with increased risk of kidney disease in HIV/HCV-coinfected persons.11 Whether injection drug use or other risk factors for HIV and HCV infections contribute to the increased risk of kidney disease has not been determined. Some studies have found an association between increased risk for kidney disease and HCV viremia and others have not: 2 secondary analyses of HIV treatment trials identified a strong association between HCV viremia and increased risk for CKD,12,13 whereas HCV infection was associated with an increased risk of CKD regardless of HCV RNA level in data from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD).14 Although improvements in kidney disease have been observed in some HIV/HCV-coinfected persons receiving HCV treatment, the impact of more widespread treatment of HCV infection on CKD risk in HIV/HCV-coinfected persons remains unclear. As discussed below, practitioners should be aware of potential drug-drug interactions between direct-acting antiviral drugs and antiretroviral therapy when treating these patients.
Treatment-Related Kidney Toxicity
The antiretroviral drugs most strongly implicated in kidney injury are protease inhibitors (in particular indinavir and atazanavir) and tenofovir disoproxil fumarate (TDF). Practitioners still report cases of CKD resulting from prior use of indinavir, although this drug is rarely used any longer. These individuals may present with scarring from chronic interstitial nephritis or obstructive nephropathy, posing increased risk for future comorbid CKD or treatment-related kidney toxicity. Although indinavir is the most strongly linked to interstitial nephritis and nephrolithiasis, all protease inhibitors are poorly soluble in urine, resulting in crystalluria that can promote renal inflammation or stone formation. The risk of nephrolithiasis may be higher with atazanavir than with other commonly used protease inhibitors, and this drug has also been associated with decreased GFR in observational studies.15,16
TDF-related kidney toxicity is generally a clinical diagnosis. A biopsy is not necessary for a person who is taking tenofovir who presents with typical tubular injury with hypophosphatemia, glycosuria, proteinuria, and an elevated creatinine level. If alternative therapy is available, the recommendation is to discontinue TDF. A biopsy to confirm TDF-related kidney toxicity is warranted in cases in which there are atypical presentations or comorbidities or in which antiretroviral therapy options are limited.
In addition to being associated with proximal tubular dysfunction, TDF may also be associated with a decrease in creatinine clearance or GFR. In randomized and cohort studies, declines in creatinine clearance were greater with TDF-containing regimens than with regimens that did not contain TDF.17 In a study in the US Veterans Affairs population, the hazard ratio for developing an estimated GFR below 60 mL/min/1.73 m2 when taking a TDF-containing regimen was statistically significant for all subgroups except for persons with preexisting CKD or diabetes, conditions which are likely stronger determinants of developing a low GFR.15 In addition to TDF, the protease inhibitors indinavir, atazanavir, and ritonavir-boosted lopinavir have been associated with CKD risk that increases with cumulative exposure.16 This increased risk has not been observed with other boosted protease inhibitors or with abacavir.
Drug-drug interactions may also increase the risk of TDF-related kidney toxicity by increasing exposure to tenofovir. In particular, the pharmacoenhancers ritonavir and cobicistat are both known to increase plasma concentrations of tenofovir. Vigilance is also warranted when using TDF with newer treatments for HCV infection. Tenofovir levels have been shown to increase when TDF is used concurrently with coformulated (/) ledipasvir/sofosbuvir with or without a ritonavir-boosted protease inhibitor.
In addition to the risk in HIV-infected persons, much remains to be learned about the potential for cumulative kidney toxicity in HIV-seronegative persons taking a TDF-containing regimen long term as HIV preexposure prophylaxis; available evidence suggests a small but statistically significant decrease in creatinine clearance or GFR but an absence of overt kidney toxicity.18,19
The newer prodrug tenofovir alafenamide (TAF) may reduce the risk of kidney toxicity compared with TDF. TAF results in lower plasma concentrations of tenofovir than TDF, which is anticipated to result in a lower risk of exposure-dependent kidney and bone toxicities. Premarketing clinical trials have demonstrated a favorable effect of TAF versus TDF on biomarkers of subclinical kidney injury or proximal tubular dysfunction, although adverse clinical events were very rare regardless of treatment assignment.20–22 In a small open-label study among individuals with creatinine clearance of 30 to 69 mL/min who were switched to a TAF-containing regimen, similar improvements in urine biomarkers were observed among those whose initial regimen included TDF. Two participants in the study discontinued TAF during the follow-up period because of decreased creatinine clearance, although both had traditional risk factors for progressive kidney disease.23 Because tenofovir levels are increased following TAF administration in the setting of decreased GFR, individuals in this setting should be monitored for potential drug-related kidney toxicity until ongoing studies confirm the safety of long-term use in this population. The safety of TAF in individuals with a prior history of TDF-related kidney toxicity has not been studied.
Management of ESRD in HIV-Infected Persons
Persons with well-controlled HIV infection and ESRD are candidates for both hemodialysis and peritoneal dialysis and should be evaluated for kidney transplantation. Early discussion and planning is important so that use of hemodialysis catheters can be avoided, as HIV-infected persons are at similar, if not higher, risk for infection and other complications related to catheter use. HIV-infected kidney transplant recipients are at risk for substantial drug-drug interactions, and any change in antiretroviral therapy should be communicated to the transplant team immediately. Based on promising data from South Africa,24 an ongoing US study will evaluate the safety of kidney transplantation from HIV-infected donors to HIV-infected recipients. If successful, this approach could reduce the wait time for kidney transplantation for HIV-infected persons, which may exceed 5 years in some urban areas.
Footnotes
Presented by Dr Wyatt in December 2015. First draft prepared from transcripts by Matthew Stenger. Reviewed and edited by Dr Wyatt in February 2017.
Financial affiliations in the past 12 months: Dr Wyatt has no relevant financial affiliations to disclose.
References
- 1. Inker LA, Wyatt C, Creamer R, et al. Performance of creatinine and cystatin C GFR estimating equations in an HIV-positive population on antiretrovirals. JAIDS. 2012; 61(3): 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gagneux-Brunon A, Delanaye P, Maillard N, et al. Performance of creatinine and cystatin C-based glomerular filtration rate estimating equations in a European HIV-positive cohort. AIDS. 2013; 27(10): 1573–1581. [DOI] [PubMed] [Google Scholar]
- 3. Bhasin B, Lau B, Atta MG, et al. HIV viremia and T-cell activation differentially affect the performance of glomerular filtration rate equations based on creatinine and cystatin C. PLoS One. 2013; 8(12): e82028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi AI, Li Y, Parikh C, Volberding PA, Shlipak MG. Long-term clinical consequences of acute kidney injury in the HIV-infected. Kidney Int. 2010; 78(5): 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y, Shlipak MG, Grunfeld C, Choi AI. Incidence and risk factors for acute kidney injury in HIV infection. Am J Nephrol. 2012; 35(4): 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nadkarni GN, Patel AA, Yacoub R, et al. The burden of dialysis-requiring acute kidney injury among hospitalized adults with HIV infection: a nationwide inpatient sample analysis. AIDS. 2015; 29(9): 1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franceschini N, Napravnik S, Eron J, Jr., Szczech LA, Finn WF. Incidence and etiology of acute renal failure among ambulatory HIV-infected patients. Kidney Int. 2005; 67(4): 1526–1531. [DOI] [PubMed] [Google Scholar]
- 8. Lucas GM, Ross MJ, Stock PG, et al. Executive summary: clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014; 59(9): 1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Medapalli RK, Parikh CR, Gordon K, et al. Comorbid diabetes and the risk of progressive chronic kidney disease in HIV-infected adults: data from the Veterans Aging Cohort Study. JAIDS. 2012; 60(4): 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mallipattu SK, Liu R, Zhong Y, et al. Expression of HIV transgene aggravates kidney injury in diabetic mice. Kidney Int. 2013; 83(4): 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wyatt CM, Malvestutto C, Coca SG, Klotman PE, Parikh CR. The impact of hepatitis C virus coinfection on HIV-related kidney disease: a systematic review and meta-analysis. AIDS. 2008; 22(14): 1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mocroft A, Neuhaus J, Peters L, et al. Hepatitis B and C coinfection are independent predictors of progressive kidney disease in HIV-positive, antiretroviral-treated adults. PLoS One. 2012; 7(7): e40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peters L, Grint D, Lundgren JD, et al. Hepatitis C virus viremia increases the incidence of chronic kidney disease in HIV-infected patients. AIDS. 2012; 26(15): 1917–1926. [DOI] [PubMed] [Google Scholar]
- 14. Lucas G, Jing Y, Sulkowski M, et al. Hepatitis C co-infection and the risk of chronic kidney disease in HIV+ individuals: Does hepatitis C Viremia matter? [Abstract 718]. Conference on Retroviruses and Opportunistic Infections. March 3–6, 2013; Atlanta, Georgia.
- 15. Scherzer R, Estrella M, Li Y, Deeks SG, Grunfeld C, Shlipak MG. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012; 26:867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mocroft A, Kirk O, Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010; 24(11): 1667–1678. [DOI] [PubMed] [Google Scholar]
- 17. Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010; 51(5): 496–505. [DOI] [PubMed] [Google Scholar]
- 18. Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015; 175(2): 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solomon MM, Lama JR, Glidden DV, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS. 2014; 28(6): 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015; 385(9987): 2606–2615. [DOI] [PubMed] [Google Scholar]
- 21. Wohl D, Oka S, Clumeck N, et al. Brief report: a randomized, double-blind comparison of tenofovir alafenamide versus tenofovir disoproxil fumarate, each coformulated with elvitegravir, cobicistat, and emtricitabine for initial HIV-1 treatment: week 96 results. JAIDS. 2016; 72(1): 58–64. [DOI] [PubMed] [Google Scholar]
- 22. Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016; 16(1): 43–52. [DOI] [PubMed] [Google Scholar]
- 23. Pozniak A, Arribas JR, Gathe J, et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48-week results from a single-arm, multicenter, open-label phase 3 study. JAIDS. 2016; 71(5): 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muller E, Barday Z, Mendelson M, Kahn D. HIV-positive-to-HIV-positive kidney transplantation–results at 3 to 5 years. N Engl J Med. 2015; 372(7): 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]


