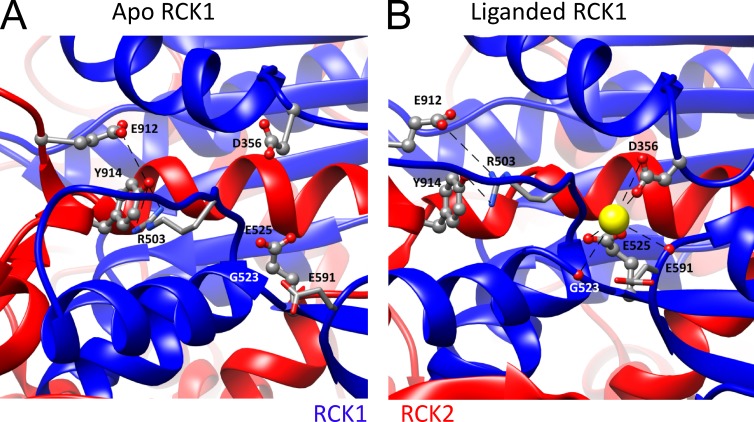
Figure 6.
The RCK1 Ca2+-binding sites. (A) High-resolution view of the metal-free structure surrounding the RCK1 Ca2+-binding sites with coordinating residues labeled. Structures in A and B were aligned by the RCK2 αS helix that resides in the vicinity of the RCK1 binding site, but appears to undergo no major change between structures. (B) High-resolution view of the liganded aSlo1 structure surrounding the RCK1 binding site with coordination mediated by backbone carbonyls of aR503 (mR514), aG523 (mS533), and aE591 (mS600) and side chains of aD356 (mD367) and aE525 (mE535). Electrostatic interaction between aE912/aY914 near the Ca2+ bowl of the same subunit with the side chain of aR503 is proposed to underlie potential interactions between RCK1 and Ca2+ bowl binding sites.