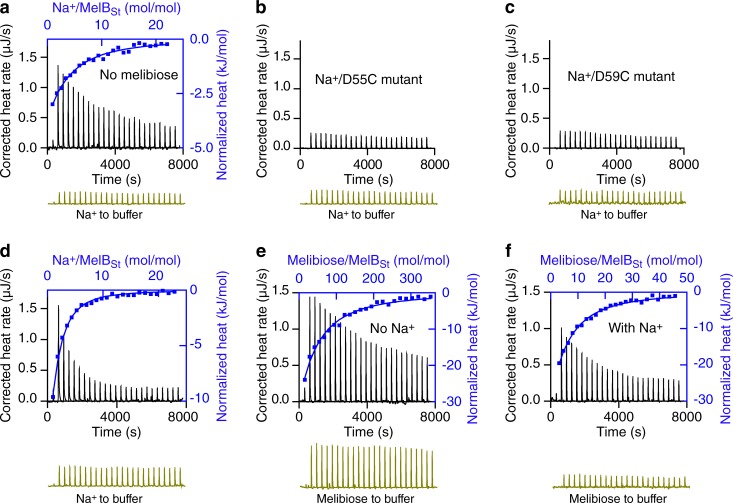
Figure 3.
Cooperative binding of Na+ and melibiose to MelBSt. (a–f) ITC was used to determine the binding of Na+ (a–d) or melibiose (e and f) to MelBSt at 25°C. Data collection was performed with a Nano ITC instrument at 25°C as described in Materials and methods. Titrant and titrand samples were subjected to buffer matching and degassing before each test. The samples containing MelBSt (a and d–f), or MelBSt mutants D55C (b) or D59C (c), at a protein concentration of 80 µM were placed in the sample cell. NaCl samples (5 mM) in the absence or presence of 50 mM melibiose placed in the syringe were injected into the MelBSt samples in the absence or presence of 50 mM melibiose or corresponding buffers without protein (controls, bottom of each panel, dark yellow). The melibiose binding to MelBSt was measured in the absence or presence of 100 mM NaCl by placing melibiose solutions (10–80 mM) in the syringe. The normalized heat changes (kJ/mol) were plotted against the Na+/MelBSt (a and d; top/right axis, blue curves) or melibiose/MelBSt molar ratio (e and f; top/right axis, blue curves), and fitted with a one-site independent-binding model with fixed stoichiometry (n = 1).