Abstract
The presence of bacteria in the lower airways in COPD results in inflammation, further airway structural damage, and might lead to repeated exacerbations. We have previously shown that chronic colonization of Haemophilus influenzae during stable disease is related to increased inflammation, and we now aimed to relate previous findings of bacterial colonization and inflammation to the degree of radiological findings of bronchiectasis and emphysema. Thirty-nine patients with COPD were included in their stable state, and a high-resolution computed tomography of the lung was performed. They were followed-up monthly for up to a maximum of 6 months or until exacerbation, and they answered questionnaires, performed spirometry, and induced sputum at every visit. Thirty-five patients had emphysema with an emphysema degree of median 20% (interquartile range 10–50), and five patients had bronchiectasis, of which only four could expectorate sputum. The degree of emphysema correlated with several inflammatory mediators in sputum, such as interleukin-8 concentration, myeloperoxidase activity, and Leukotriene B4 concentration. Ten patients were chronically colonized with H. influenzae (ie, had a positive culture for H. influenzae at all visits). The four sputum patients with bronchiectasis were chronically colonized with H. influenzae and showed higher degree of H. influenzae growth compared to patients without bronchiectasis. During exacerbation, there was no longer any correlation between emphysema degree and inflammation, but patients with bronchiectasis showed higher sputum purulence score than patients without bronchiectasis. Emphysema and bronchiectasis in COPD patients show different clinical features. The presence of emphysema is more related to inflammation, while bronchiectasis is associated with bacterial colonization. We believe that both emphysema and bronchiectasis are therefore COPD phenotypes of highest impact and need evaluation to prevent further disease progression.
Keywords: bronchiectasis, emphysema, COPD, chronic colonization, inflammation, pathogenic bacteria
Introduction
COPD is a heterogeneous disease, and its clinical features vary greatly among patients despite having a similar reduction in lung function. It is important to identify patient groups with unique specific characteristics to guide therapy and management of COPD.
Emphysema is one of the phenotypes in COPD and its degree of spreading can vary and be unevenly distributed over the lung. Patients with emphysema have more severe lung function impairment and poorer quality of life.1 Emphysema is associated with increased activity of proteolytic enzymes, which are activated due to inflammation and oxidative stress occurring in COPD,2 and emphysema degree has been shown to correlate with the ratio of interleukin (IL-8)/tissue inhibitor of metalloproteinase-1.3
Bronchiectasis is another feature of COPD, and presence of bronchiectasis in COPD is associated with increased risk of exacerbation, isolation of a potentially pathogenic microorganism, severe airway obstruction, and mortality.4,5 In addition, it has been shown that COPD patients with bronchiectasis had longer recovery time after exacerbation and higher levels of inflammatory cytokines.6,7 The prevalence of bronchiectasis in COPD is reported to range between 29% and 68%,7–10 which is most probably dependent on patient selection of COPD severity and sputum productivity.
Bacterial colonization in COPD is an important factor in the disease progression,11 and some COPD patients are colonized by bacteria during stable phase between exacerbations.12 In particular, it may lead to a persistent airway inflammation even in the stable clinical state, which may contribute to morbidity of the disease. Even in the absence of clinical exacerbation, colonization by bacterial pathogens in stable COPD was associated with an increase in daily symptom13 and an accelerated decline in forced expiratory volume in 1 s (FEV1).14 Haemophilus influenzae has been shown to be the most prevalent individual bacterial type in stable COPD patients12,14,15 as well as specifically in patients with bronchiectasis.8,16,17 Colonization with H. influenzae was also found repeatedly in COPD patients with bronchiectasis.8,17
Bacterial colonization is a pathogenic factor related to COPD progression, and we have previously shown that H. influenzae has evidence of more active disease with increased inflammation, and specifically in patients with repeated (chronic) colonization during stable disease.12 There is no long-term prospective study investigating bacterial colonization in patients during repeated visits in relation to bronchiectasis and emphysema. In the present study, we therefore aimed to relate previous findings of inflammation and bacterial colonization to radiological phenotype, including bronchiectasis and emphysema degree, by using high-resolution computed tomography (HRCT).
Materials and methods
Subjects
Thirty-nine patients with COPD, with at least one antibiotic demanding exacerbation during the past year, were included (Table 1). They were diagnosed according to global initiative for chronic obstructive lung disease (GOLD).18 All patients were consecutive patients who came for checkup at the Lung Clinic at Lund University Hospital, Sweden. Study participants had no history of lung cancer or asthma. Additionally, the subjects were free from exacerbation treatment 3 weeks prior to the study. The patients were not allowed to have antibiotics treatment or oral corticosteroids during the study. All subjects, but one, were on inhaled corticosteroids (middle-high dose) and either long-acting or short-acting beta2-agonists, as well as either long-acting or short-acting muscarinic antagonists (Table 1).
Table 1.
Patient characteristics
| COPD patients, n=39 | |
|---|---|
| Age (years) | 65 (61–69) |
| Females/males (n) | 22/17 |
| Current/former smokers (n) | 8/31 |
| Pack years | 40 (35–50) |
| GOLD stage 1/2/3/4 (n) | 1/13/16/9 |
| ICS (n) | 38 |
| LABA/SABA (n) | 37/28 |
| LAMA/SAMA (n) | 26/26 |
Note: Age and pack-years are given as median (interquartile range), and the other parameters are given as n of respective subgroup.
Abbreviations: GOLD, global initiative for chronic obstructive lung disease; ICS, inhaled corticosteroids; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting beta2-agonist; SAMA, short-acting muscarinic antagonist.
Study design
Study participants were included in their stable state and were followed-up monthly for up to a maximum of 6 months. The patients came for an extra visit (prior to scheduled visit) when exacerbating.19 An exacerbation was defined according to GOLD guidelines.18 The study was approved by the Regional Ethical Review Board in Lund (Dnr 634/2005), and all study participants signed written informed consent. All patients were subjected to an HRCT; most patients within the time of the study (n=28) or within 6 months before or after the study (n=7), while four patients were subjected to an HRCT 11 months before or 17, 18, and 30 months after the study, respectively. A physical examination was performed before the study started. During all visits, patients inhaled 5 mg salbutamol (Ventolin™, GlaxoSmithKline plc, London, UK) and 0.5 mg ipratropium (Atrovent™, Boehringer Ingelheim, Ingelheim, Germany) prior to all investigations. The patients filled in two questionnaires: Clinical COPD Questionnaire (CCQ)20 and Medical Research Council (MRC) dyspnea scale. Thereafter, spirometry and sputum induction were performed. Each parameter value of stable phase was calculated as an average value of all visits during stable phase.
Spirometry
All subjects performed spirometry (using Jaeger Master-Screen, Erich Jaeger GmbH, Würzburg, Germany). Reference values established by Crapo et al were used.21
HRCT and image analysis
The scanning, covering the whole lung, was performed with the patients in supine position. They were, with few exceptions, performed on a Philips Mx 8000 IDT 16 or a Brilliance 40 slice CT (Philips Medical Systems, Best, the Netherlands) with tube voltage of 120 and 140 kV, exposure of 240 and 288 mAs, respectively, and high-resolution mode and high-resolution filter with reconstructed slice thickness of 1.0–10.0 mm. The examinations were performed in a clinical setting and reviewed retrospectively. The images were analyzed in consensus by two chest radiologists blinded to clinical data and using a clinical PACS workstation (Sectra IDS 7) with generally accepted window settings for lung parenchyma (center −500 HU and width 1,500 HU). HRCT images were assessed with focus on the presence of emphysema, bronchiectasis, and bronchial wall thickening.
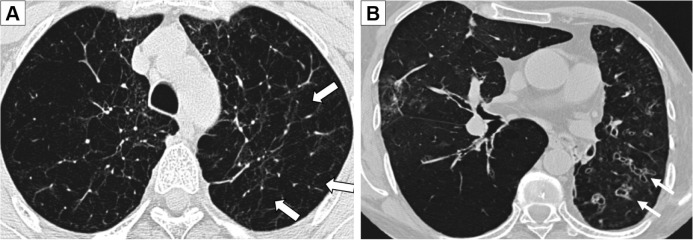
Emphysema (Figure 1A) was defined according to the Fleischner Society Glossary of Thoracic imaging,22 and the extent was estimated as a percentage of the total lung volume in 10% step size, modified from already existing methods.23 Bronchiectasis (Figure 1B) was also defined according to the Fleischner Society Glossary of Thoracic imaging,22 and if present scored into mild, moderate, and severe if the number of bronchiectasis was 1–5, 6–9, or above as adapted from Lee et al.24 For bronchial wall thickening, there is no precise definition, and visual estimation is subjective.25,26 The scoring was estimated to be none, mild, moderate, or severe with reference images.27
Figure 1.
HRCT pictures showing emphysema (A) in the upper lobes (white arrows) and severe bronchiectasis (B) with bronchial wall thickening (white arrows).
Abbreviation: HRCT, high-resolution computed tomography.
Sputum induction, processing, and analysis
Sputum was induced in all patients at all visits by inhalation of nebulized 0.9% NaCl for 0.5, 1, 2, and 4 min, followed by 4.5% NaCl for 0.5, 1, 2, and 4 min. Sputum induction was continued until adequate sample volume was obtained or until full time according to the protocol.
Sputum purulence was determined using a colorimetric scale.28 One aliquot of the sputum was sent for bacterial culture at the Clinical Microbiology Department, Lund University Hospital, and analyzed according to clinical routine. Pathogen growth was classified into low, moderate, and high degree of growth.
Sputum plugs were sorted out from the second aliquot, treated with four volumes of 0.65 mM dithiothreitol (DTT) in phosphate-buffered saline (PBS) for 1 h in 4°C, followed by the addition of four volumes of PBS. The sputum was filtered (60 µm), centrifuged (1,000× g for 5 min), and the supernatant was frozen for later analysis.29,30
Sputum samples were analyzed for Leukotriene B4 (LTB4) using an EIA kit from Cayman Chemical (Ann Arbor, MI, USA). Before analysis of subsequent protein assays, sputum was dialyzed in PBS to eliminate the remaining DTT. IL-8 was measured using Quantikine (R&D Systems, Inc., Minneapolis, MN, USA). MPO activity was measured according to Axelsson et al,31 by adding 3,3′,5,5′-tetramethylbenzidine and hydrogen peroxide (H2O2) to the sputum, stopping the reaction with 2 M H2SO4 after 3 min, and analyzing at 450 nm. Horseradish peroxidase was used as standard.
All values were adjusted to the total amount of protein in sputum (measured using the Bio-Rad Protein Assay [Bio-Rad Laboratories, Inc., Hercules, CA, USA]) and presented as amount per microgram total protein to abolish differences due to sputum heterogeneity.
Statistics
Statistical comparison between separate groups was performed with Mann–Whitney’s U-test for unpaired samples. Spearman’s correlation test was used for correlation analyses, and linear regression analyses were performed in correction analyses. Chi-square test was used for group comparisons. All statistical calculations were performed using SPSS 20.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the median (interquartile range [IQR]). The level of significance was set at P≤0.05.
Results
Patients
Thirty-five patients (90%) had emphysema with an emphysema degree of median 20% (IQR 10–50). Of the emphysema patients, 31 patients could expectorate sputum during stable phase, and 21 did an exacerbation visit with successful expectoration of sputum. Five patients (13%) showed bronchiectasis on HRCT (four mild and one severe, all having emphysema), of which only four could expectorate sputum during stable phase, and additionally one during exacerbation. A total of 36 of the patients (including all patients with bronchiectasis) showed bronchial wall thickening (21 mild, 13 moderate, and 2 severe).
The eight smokers all had emphysema, but there was no difference in emphysema degree between smokers and nonsmokers. In contrast, only two of the smokers had bronchiectasis.
A total of 187 patient visits were performed in this study and a median (IQR) of five (3–7) visits were performed per patient (either until exacerbation or until 6 months). From nine patients, no adequate sputum could be obtained during stable phase, but five of them could expectorate sputum during exacerbation.
Degree of emphysema relates to inflammation during stable phase
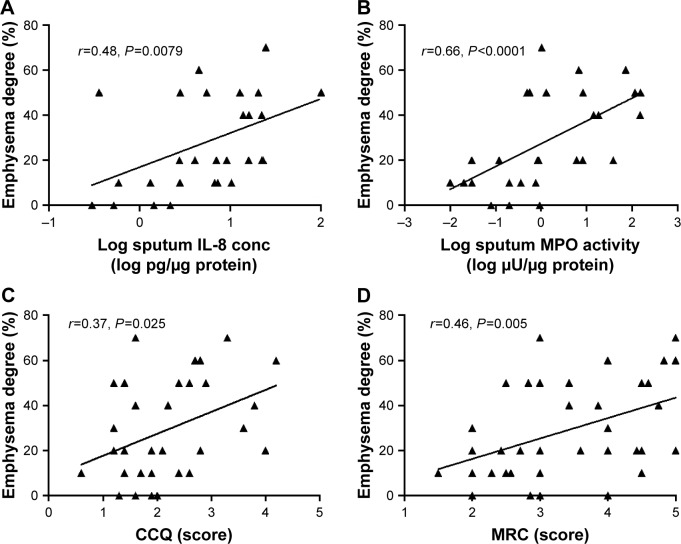
The degree of emphysema correlated with several inflammatory mediators in sputum, such as IL-8 concentration (Figure 2A), MPO activity (Figure 2B), and LTB4 concentration (r=0.37, P=0.050). In addition, the degree of emphysema correlated with FEV1 (r=−0.41, P=0.010), FEV1%p (r=−0.36, P=0.030), and FEV1/forced vital capacity (FVC) (r=−0.36, P=0.027), but not to FVC (r=−0.15, P=0.38) or FVC%p (r=−0.15, P=0.36). In addition, the degree of emphysema correlated with dyspnea, according to CCQ score (Figure 2C) and MRC score (Figure 2D). After correction with FEV1 or FEV1%p, the correlation between degree of emphysema and inflammatory mediators in sputum was no longer significant. However, the correlation between degree of emphysema and dyspnea was still significant after correction for FEV1 (CCQ: P=0.009, MRC: P=0.016) or FEV1%p (CCQ: P=0.009, MRC: P=0.022).
Figure 2.
The correlation between emphysema degree and sputum biomarkers (log IL-8 concentration [A, n=29] and log MPO activity [B, n=28]) and symptom score (CCQ [C, n=37] and MRC [D, n=37]) in COPD patients during stable phase (using the mean of each parameter at all stable visits).
Note: Data are presented as individual value for each patient, and Spearman’s r and P values are shown in the respective figure.
Abbreviations: CCQ, Clinical COPD Questionnaire; conc, concentration; IL, interleukin; MPO, myeloperoxidase; MRC, Medical Research Council.
Bacterial colonization during stable phase
Thirteen patients were colonized with the same bacteria at all their visits during the stable phase of the study (= chronically colonized). The definition was due to all sputum samples from the same patients, and no minimum number of positive sputum samples was set. Ten patients were chronically colonized with H. influenzae (median number of visits was 4 [IQR 3–7]), two with Staphylococcus aureus and one with Pseudomonas aeruginosa.
Twelve patients did not show any positive culture of pathogenic bacteria (H. influenzae, S. aureus, P. aeruginosa, Streptococcus pneumoniae, or Moraxella catarrhalis) at any visit during this study. No patients were chronically colonized with S. pneumoniae or M. catarrhalis.
Emphysema degree in relation to chronic bacterial colonization during stable phase
In patients with emphysema, 10 had chronic colonization with H. influenzae, while 17 had not. None of the four patients without emphysema had chronic colonization with H. influenzae (P=0.15, Table 2). However, the degree of emphysema was higher in patients with chronic colonization with H. influenzae, compared to patients with no chronic colonization of H. influenzae (P=0.025, Figure 3).
Table 2.
Radiological phenotypes of COPD patients (that could expectorate sputum) with or without chronic colonization of Haemophilus influenzae during stable phase
| Clinical characteristics | No chronic colonization with H. influenzae | Chronic colonization with H. influenzae | Chi-square |
|---|---|---|---|
| Emphysema | P=0.15 | ||
| Yes, n=27 | 17 | 10 | |
| No, n=4 | 4 | 0 | |
| Bronchiectasis | P=0.008 | ||
| Yes, n=4 | 0 | 4 | |
| No, n=27 | 21 | 6 | |
| Bronchial wall thickening | P=0.071 | ||
| Yes, n=28 | 19 | 9 | |
| No, n=3 | 2 | 1 |
Note: Bold number depicts significant value.
Figure 3.
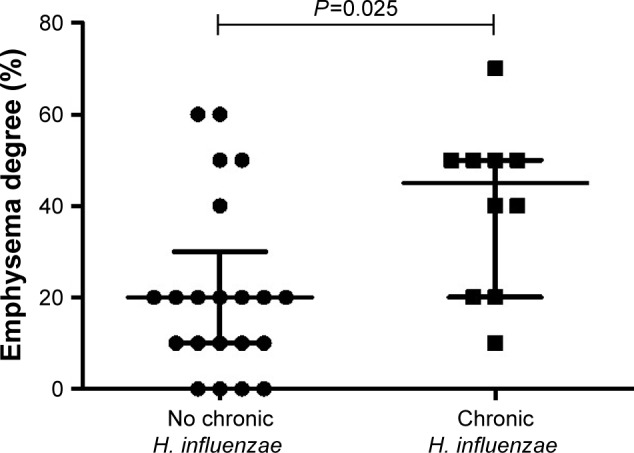
The emphysema degree measured by HRCT in COPD patients with (n=10) or without (n=21) chronic colonization (= colonization at all visits) of Haemophilus influenzae in sputum during stable phase.
Notes: Data are presented as individual dots and bars for median and IQR. P-value for statistical significance between colonized and not colonized patients is shown in the figure.
Abbreviations: HRCT, high-resolution computed tomography; IQR, interquartile range.
Bronchiectasis in relation to chronic bacterial colonization during stable phase
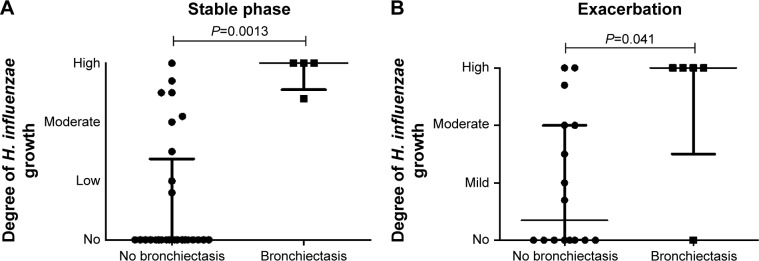
Interestingly, all patients with bronchiectasis had chronic colonization with H. influenzae, while among those without bronchiectasis, 6 had chronic colonization with H. influenzae, while 21 had not (P=0.008, Table 2). In addition, those patients with bronchiectasis had a higher degree of H. influenzae growth during stable phase compared to patients without bronchiectasis (P=0.0013, Figure 4A).
Figure 4.
The degree of Haemophilus influenzae growth in sputum during stable phase (A) and exacerbation (B) in COPD patients with (n=4 during stable phase and n=5 during exacerbation) or without (n=27 during stable phase and n=16 during exacerbation) bronchiectasis according to HRCT. H. influenzae growth is classified as none, mild, moderate, and high due to routine culture at the Clinical Microbiology Department, Lund University Hospital.
Notes: Data are presented as individual dots and bars for median and IQR. P-values for statistical significance between COPD patients with or without bronchiectasis are shown in the figure.
Abbreviations: HRCT, high-resolution computed tomography; IQR, interquartile range.
Degree of emphysema and inflammation during exacerbation
Twenty-three patients (59%) achieved an exacerbation within the 6 months period at median visit 3 (IQR 2–5). Five patients did not manage to come for the exacerbation visit or could not expectorate any sputum.
During exacerbation, the emphysema degree did no longer correlate with inflammatory mediators in sputum, such as IL-8 concentration, MPO activity, and LTB4 concentration (all P>0.57).
Bronchiectasis and bacterial colonization during exacerbation
Of the 18 patients who expectorated sputum during exacerbation, 14 patients were colonized with pathogenic bacteria at exacerbation (10 patients had H. influenzae colonization, 3 had S. aureus, 2 had P. aeruginosa, 4 had S. pneumonia, and 4 had M. catarrhalis during exacerbation). Similar to stable phase, patients with bronchiectasis still had significantly higher degree of H. influenzae growth during exacerbation (P=0.041, Figure 4B).
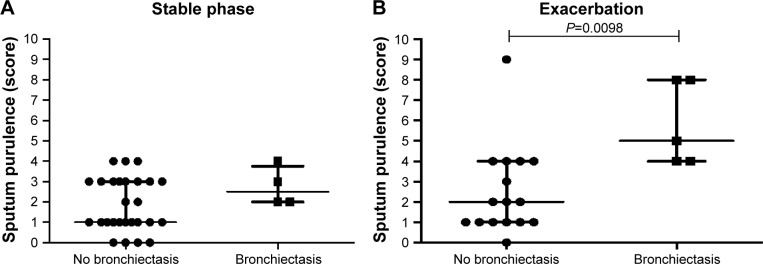
During exacerbation the sputum purulence was higher in COPD patients with bronchiectasis compared to those not having any bronchiectasis (P=0.012, Figure 5B), which was not seen during stable phase (Figure 5A).
Figure 5.
Sputum purulence scores during stable phase (A) and exacerbation (B) in COPD patients with (n=4 during stable phase, and n=5 during exacerbation) or without (n=27 during stable phase, and n=16 during exacerbation) bronchiectasis according to HRCT.
Notes: Data are presented as individual dots and bars for median and IQR. P-value for statistical significance between COPD patients with or without bronchiectasis is shown in the figure.
Abbreviations: HRCT, high-resolution computed tomography; IQR, interquartile range.
Discussion
After following COPD patients monthly during their stable phase, we show that higher emphysema degree is related to more inflammation measured in sputum and that patients chronically colonized with H. influenzae have higher emphysema degree. In addition, all patients with bronchiectasis were chronically colonized with H. influenzae and showed higher degree of H. influenzae growth compared to patients without bronchiectasis. During exacerbation, there was no longer any correlation between emphysema degree and inflammation, but patients with bronchiectasis showed higher sputum purulence score than patients without bronchiectasis.
Bronchiectasis and emphysema lead to distinctive clinical features. The presence of bronchiectasis in COPD patients has a greater correlation with the parameters of patients with COPD with chronic bronchitis phenotype (thicker bronchial wall, greater daily sputum production, and a higher number of exacerbations) than those with the emphysema phenotype.32
Most of the patients had emphysema to some degree, and a correlation between emphysema degree and inflammation measured in sputum during stable phase suggests that severity of the emphysema is related to an established stable inflammatory phenotype. However, during exacerbation, there is no longer any correlation between emphysema degree and these inflammatory biomarkers, suggesting further addition of distinguished inflammatory progress.
We also confirm a relationship between emphysema degree and symptom score (MRC and CCQ) during stable phase, which has previously been suggested.1,33 Unfortunately, only a few patients had bronchiectasis so its relationship to inflammation could not be investigated. Interestingly, the relationship between degree of emphysema and dyspnea was still significant after correction for FEV1 or FEV1%p, which was not the case for inflammatory mediators in sputum. This suggests that dyspnea might be related to the emphysema itself, while inflammatory mediators might be more related to the severe airflow obstruction.
Patients with bronchiectasis have increased C-reactive protein, which is most probably due to colonization of pathogens.34 Repeated colonization of especially H. influenzae can be seen in COPD patients with bronchiectasis,8 and we have previously shown that many of these patients have chronic colonization of H. influenzae, which in turn was related to increased inflammation measured in sputum.12 After investigation of radiological phenotype of these patients, we can conclude that patients with bronchiectasis all have chronic colonization of H. influenzae and also a high degree of H. influenzae growth. Even though only a few patients had bronchiectasis, these findings were significant. A similar relationship between H. influenzae and presence of emphysema was not found, which is in accordance with a previous publication showing no relationship between the degree of emphysema on HRCT and bacterial colonization.7
A change in the microbial flora has been shown with increasing severity of COPD, and H. influenzae becomes more predominant as lung function declines.17 H. influenzae is often present in the lungs of patients with end-stage pulmonary disease, especially cystic fibrosis and COPD patients.35 This is in accordance with the present study investigating a cohort with relatively severe COPD, where H. influenzae is the most abundant pathogenic bacteria. The number of patients with bronchiectasis is relatively low, but within the range of previously published numbers.7–10,36
Bronchiectasis patients have been shown to have more exacerbation indices.8 However, another study showed that the number of exacerbations was not related to bronchiectasis, but patients with bronchiectasis rather experienced longer exacerbations.7 In this study, we have too few patients with bronchiectasis to be able to draw any conclusion on this matter. Though a subinvestigation of patients with increased emphysema degree (either above median [20%] or above 40%) or high bacterial colonization (above median) compared to patients with lower values showed that there were no differences in gender, exacerbation, smoking status, or medication.
Patients with bronchiectasis are more likely to produce purulent sputum continually (continual purulent sputum) than those without bronchiectasis.10 In our study, there was no difference in sputum purulence score in patients with or without bronchiectasis during stable phase, but the sputum purulence score was higher during exacerbation, suggesting a link to an established underlying bacterial infection that progresses during exacerbation. This was not affected by smoking status, since the two of the bronchiectasis patients were smokers and did not show any particular pattern.
The presence of bacteria in the lower airways in COPD impairs host defense mechanisms, which results in inflammation and further airway structural damage. For example, H. influenzae is associated with higher sputum levels of neutrophil elastase and IL-1β.37 This could be the mechanism for repeated exacerbations, and incomplete resolution of bacterial colonization is considered to be a risk for relapse. This phenomenon of chronic colonization, secondary inflammatory reaction, and progressive lung damage is a vicious cycle,38 and an appropriate evaluation of airway colonization is needed to prevent further disease progression. Subsequently, appropriate and effective antibiotic therapy during exacerbation of COPD patients with bronchiectasis may therefore be important in preventing exacerbations and relapses.
The reviewing of the images was done in consensus in order to better estimate the extent of emphysema. Using consensus the interobserver variability was not addressed which is unfortunate. However, this has been previously reported in other studies.39 With the image analysis performed retrospectively, it is possible that the differences in image quality may have affected the result. Most patients were subjected to HRCT within the time of the study or within 6 months before or after the study. However, four of the patients had longer time between the study and the HRCT. None of the four patients had bronchiectasis, and all had average levels of emphysema degree. This is, though, a limitation of the study that might have influenced the results.
Conclusion
We show that more inflammation measured in sputum during stable phase is related to a higher emphysema degree in COPD patients and that patients chronically colonized with H. influenzae have higher emphysema degree. In addition, all patients with bronchiectasis were chronically colonized with H. influenzae and showed a higher degree of H. influenzae growth compared to patients without bronchiectasis. Emphysema and bronchiectasis in COPD patients seem to be different clinical features but are often combined. The presence of emphysema is more related to inflammation, while bronchiectasis is associated with bacterial colonization. We believe that both emphysema and bronchiectasis are therefore COPD phenotypes of highest impact.
Acknowledgments
This work was supported by independent research grants from the Swedish Heart and Lung foundation, Swedish Research Council, Evy and Gunnar Sandberg’s Foundation, and Royal Physiographic Society in Lund. We thank Anna-Karin Juhlin, Harriet Johansson, Tove Alvå, and Oskar Ask at the Lung Clinic, Skåne University Hospital, Lund for highly appreciated clinical assistance.
Footnotes
Author contributions
ET has designed the study, performed the analysis, interpreted data, and written the manuscript. HM and GB have performed HRCT analyses and critically revised the manuscript; ME has designed the study, included patients, interpreted data, and critically revised the manuscript; and LB has designed the study, interpreted data, and critically revised the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interests in this work.
References
- 1.Papaioannou AI, Mazioti A, Kiropoulos T, et al. Systemic and airway inflammation and the presence of emphysema in patients with COPD. Respir Med. 2010;104(2):275–282. doi: 10.1016/j.rmed.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Angelis N, Porpodis K, Zarogoulidis P, et al. Airway inflammation in chronic obstructive pulmonary disease. J Thorac Dis. 2014;6(suppl 1):S167–S172. doi: 10.3978/j.issn.2072-1439.2014.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaker SB, von Wachenfeldt KA, Larsson S, et al. Identification of patients with chronic obstructive pulmonary disease (COPD) by measurement of plasma biomarkers. Clin Respir J. 2008;2(1):17–25. doi: 10.1111/j.1752-699X.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 4.Du Q, Jin J, Liu X, Sun Y. Bronchiectasis as a comorbidity of chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0150532. doi: 10.1371/journal.pone.0150532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni Y, Shi G, Yu Y, Hao J, Chen T, Song H. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1465–1475. doi: 10.2147/COPD.S83910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angrill J, Agusti C, De Celis R, et al. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am J Respir Crit Care Med. 2001;164(9):1628–1632. doi: 10.1164/ajrccm.164.9.2105083. [DOI] [PubMed] [Google Scholar]
- 7.Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(4):400–407. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Garcia MA, Soler-Cataluna JJ, Donat Sanz Y, et al. Factors associated with bronchiectasis in patients with COPD. Chest. 2011;140(5):1130–1137. doi: 10.1378/chest.10-1758. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55(8):635–642. doi: 10.1136/thorax.55.8.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith IE, Jurriaans E, Diederich S, Ali N, Shneerson JM, Flower CD. Chronic sputum production: correlations between clinical features and findings on high resolution computed tomographic scanning of the chest. Thorax. 1996;51(9):914–918. doi: 10.1136/thx.51.9.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tufvesson E, Bjermer L, Ekberg M. Patients with chronic obstructive pulmonary disease and chronically colonized with Haemophilus influenzae during stable disease phase have increased airway inflammation. Int J Chron Obstruct Pulmon Dis. 2015;10:881–889. doi: 10.2147/COPD.S78748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai H, Eschberger K, Wrona C, et al. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(3):303–309. doi: 10.1513/AnnalsATS.201310-350OC. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(8):1090–1095. doi: 10.1164/rccm.200210-1179OC. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Li Q, Zhang XY, Ding X, Zhu D, Zhou X. Relevance of lower airway bacterial colonization, airway inflammation, and pulmonary function in the stable stage of chronic obstructive pulmonary disease. Eur J Clin Microbiol Infect Dis. 2010;29(12):1487–1493. doi: 10.1007/s10096-010-1027-7. [DOI] [PubMed] [Google Scholar]
- 16.Angrill J, Agusti C, de Celis R, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax. 2002;57(1):15–19. doi: 10.1136/thorax.57.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Microbiologic follow-up study in adult bronchiectasis. Respir Med. 2007;101(8):1633–1638. doi: 10.1016/j.rmed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 19.Tufvesson E, Ekberg M, Bjermer L. Inflammatory biomarkers in sputum predict COPD exacerbations. Lung. 2013;191(4):413–416. doi: 10.1007/s00408-013-9473-5. [DOI] [PubMed] [Google Scholar]
- 20.van der Molen T, Willemse BW, Schokker S, ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual Life Outcomes. 2003;1:13. doi: 10.1186/1477-7525-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123(6):659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 22.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 23.Malinen A, Erkinjuntti-Pekkanen R, Partanen K, Rytkonen H, Vanninen R. Reproducibility of scoring emphysema by HRCT. Acta Radiol. 2002;43(1):54–59. doi: 10.1080/028418502127347448. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Kim YK, Kwag HJ, Chang JH. Relationships between high-resolution computed tomography, lung function and bacteriology in stable bronchiectasis. J Korean Med Sci. 2004;19(1):62–68. doi: 10.3346/jkms.2004.19.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansell DM. Thin-section CT of the lungs: the Hinterland of normal. Radiology. 2010;256(3):695–711. doi: 10.1148/radiol.10092307. [DOI] [PubMed] [Google Scholar]
- 26.Naidich DP. Imaging of the Airways: Functional and Radiologic Correlations. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 27.Barr RG, Berkowitz EA, Bigazzi F, et al. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD. 2012;9(2):151–159. doi: 10.3109/15412555.2012.654923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allegra L, Blasi F, Diano P, et al. Sputum color as a marker of acute bacterial exacerbations of chronic obstructive pulmonary disease. Respir Med. 2005;99(6):742–747. doi: 10.1016/j.rmed.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Pavord ID, Sterk PJ, Hargreave FE, et al. Clinical applications of assessment of airway inflammation using induced sputum. Eur Respir J Suppl. 2002;37:40s–43s. doi: 10.1183/09031936.02.00004002. [DOI] [PubMed] [Google Scholar]
- 30.Tufvesson E, Aronsson D, Bjermer L. Cysteinyl-leukotriene levels in sputum differentiate asthma from rhinitis patients with or without bronchial hyperresponsiveness. Clin Exp Allergy. 2007;37(7):1067–1073. doi: 10.1111/j.1365-2222.2007.02746.x. [DOI] [PubMed] [Google Scholar]
- 31.Axelsson JB, Akbarshahi H, Said K, Malmstrom A, Andersson R. Proposed protective mechanism of the pancreas in the rat. J Inflamm (Lond) 2010;7:24. doi: 10.1186/1476-9255-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Garcia MA, de la Rosa Carrillo D, Soler-Cataluna JJ, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(8):823–831. doi: 10.1164/rccm.201208-1518OC. [DOI] [PubMed] [Google Scholar]
- 33.Bajc M, Markstad H, Jarenback L, Tufvesson E, Bjermer L, Jogi J. Grading obstructive lung disease using tomographic pulmonary scintigraphy in patients with chronic obstructive pulmonary disease (COPD) and long-term smokers. Ann Nucl Med. 2015;29(1):91–99. doi: 10.1007/s12149-014-0913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tulek B, Kivrak AS, Ozbek S, Kanat F, Suerdem M. Phenotyping of chronic obstructive pulmonary disease using the modified Bhalla scoring system for high-resolution computed tomography. Can Respir J. 2013;20(2):91–96. doi: 10.1155/2013/727523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moller LV, Timens W, van der Bij W, et al. Haemophilus influenzae in lung explants of patients with end-stage pulmonary disease. Am J Respir Crit Care Med. 1998;157(3 pt 1):950–956. doi: 10.1164/ajrccm.157.3.9707010. [DOI] [PubMed] [Google Scholar]
- 36.Okada F, Ando Y, Tanoue S, et al. Radiological findings in acute Haemophilus influenzae pulmonary infection. Br J Radiol. 2012;85(1010):121–126. doi: 10.1259/bjr/48077494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson JL, Baines KJ, Horvat JC, et al. COPD is characterized by increased detection of Haemophilus influenzae, Streptococcus pneumoniae and a deficiency of Bacillus species. Respirology. 2016;21(4):697–704. doi: 10.1111/resp.12734. [DOI] [PubMed] [Google Scholar]
- 38.Cole PJ. Inflammation: a two-edged sword – the model of bronchiectasis. Eur J Respir Dis Suppl. 1986;147:6–15. [PubMed] [Google Scholar]
- 39.Spouge D, Mayo JR, Cardoso W, Muller NL. Panacinar emphysema: CT and pathologic findings. J Comput Assist Tomogr. 1993;17(5):710–713. [PubMed] [Google Scholar]