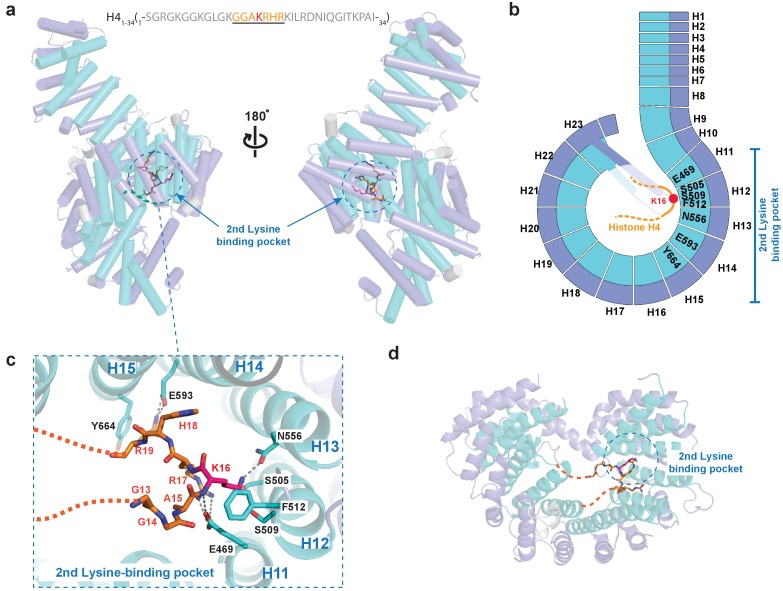
Figure 4. Crystal structure of Kl Kap123 in complex with H41–34-NLS.
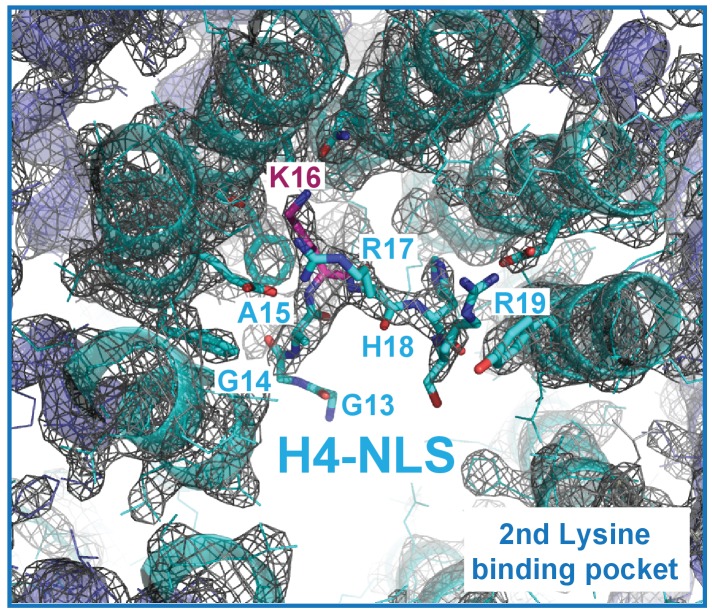
(a) The crystal structure of full-length Kl Kap123 in the presence of H41–34-NLS (orange stick model, 1-SGRGKGGKGLGKGGAKRHRKILRDNIQGITKPAI-34) with two lateral views (180° rotation). The second lysine-binding pocket located at the inner curvature of Kap123 is marked as a blue dashed circle. Residues 13–20 of H41–34-NLS are visible in the structure. H4 K16, which binds to the second lysine-binding pocket, is colored red. (b) Schematic view of Kl Kap123 in complex with H41–34-NLS. Residues of repeats 11–15 that participate in H41–34-NLS recognition are indicated. (c) The second lysine-binding pocket of Kl Kap123 in H41–34-NLS recognition. K16 of H41–34-NLS forms hydrophobic (F512) and electrostatic/hydrogen bond (S505, S509, and N556) interactions with Kap123 through repeats 12–13. R17 and R19 of H41–34-NLS form additional electrostatic and hydrophobic contacts with E469 (repeat 11) and E593 (repeat 14)/Y664 (repeat 15) of Kap123, respectively. (d) Top view of Kl Kap123 in complex with H41–34-NLS. Only the second lysine-binding pocket is occupied by K16 of H41–34-NLS.