Table 2. Coupling of Aryl Halides with Primary Alkylamines Using (CyPF-tBu)PdCl2 1a.
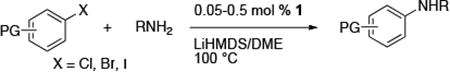
| |||||
|---|---|---|---|---|---|
| entry | halide | product | catalyst loading (%) | conditions | yield (%)b |
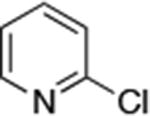
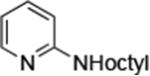
| 1 |
|
|
0.001 | 110 °C, 24 h | 92 |
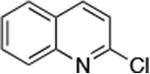
| 2 |
|
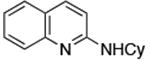
|
0.01 | 110°C,24 h | 85 |
| 3 |
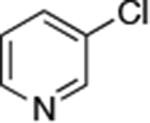
|
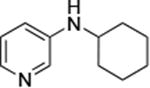
|
0.05 | 100°C,24 h | 88 |
| 4 |
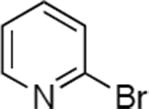
|
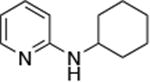
|
0.005 | 80 °C, 36 h | 86 |
| 5 |
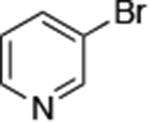
|
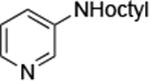
|
0.005 | 100°C,36 h | 74 |
| 6c | 0.005 | 110 °C, 12 h | 87 | ||
| 7 |
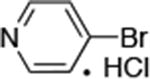
|
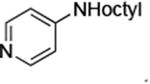
|
0.05 | 100°C,30 h | 88 |
| 8 |
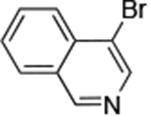
|
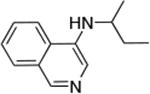
|
0.05 | 100°C,24 h | 96 |
| 9 |
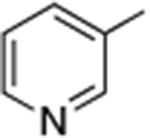
|
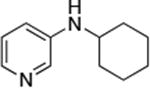
|
0.05 | 110 °C, 24 h | 70 |
Reactions conducted with a 1:1 ratio of metal to ligand, 1 mmol ArX, 1.2 equiv amine, and 1.4 equiv NaO-tBu in 1 mL DME.
Isolated yield.
