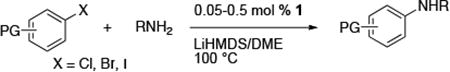
Table 3. Amination of Functionalized Aryl halides with Primary Alkylamine Using (CyPF-tBu)PdCl2 1a.
| |||||
|---|---|---|---|---|---|
| entry | halide | product | catalyst loading (%) | conditions | yield (%)b |
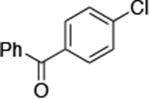
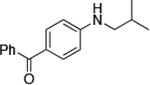
| 1 |
|
|
0.05 | 100 °C 48 h | 84 |
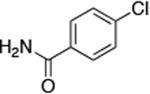
| 2 |
|
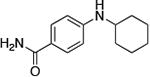
|
0.5 | 100 °C 36 h | 52 |
| 3c |
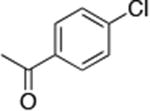
|
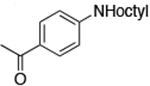
|
0.5 | 110 °C 24 h | 67 |
| 4 |
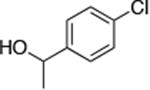
|
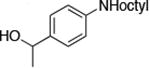
|
0.5 | 100 °C 24 h | 77 |
| 5 |
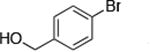
|
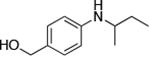
|
0.5 | 100 °C 24 h | 69 |
| 6 |
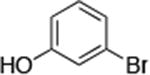
|
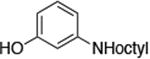
|
0.05 | 100 °C 24 h | 84 |
| 7 |
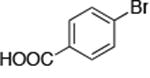
|
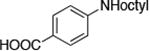
|
0.05 | 100 °C 24h | 78 |
| 8c |
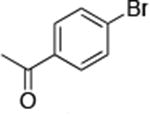
|
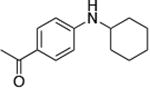
|
1.0 | 110 °C 24 h | 75 |
| 9c |
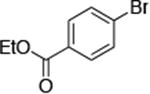
|
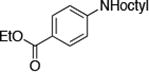
|
0.5 | 110 °C 24 h | 88 |
| 10c |
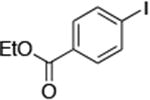
|
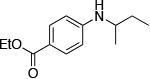
|
2.0 | 110 °C 48h | 71 |
| 11c |
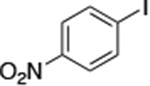
|
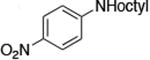
|
1.0 | 110 °C 24 h | 85 |
| 12c |
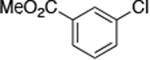
|
|
1.0 | 110 °C 24 h | 89 |
Reactions conducted with a 1:1 ratio of metal to ligand, 1 mmol ArX, 1.2 equiv amine, and 2.4 equiv LiN(SiMe3)2 in 1 mL DME.
Isolated yield.
Using K3PO4 as the base.
