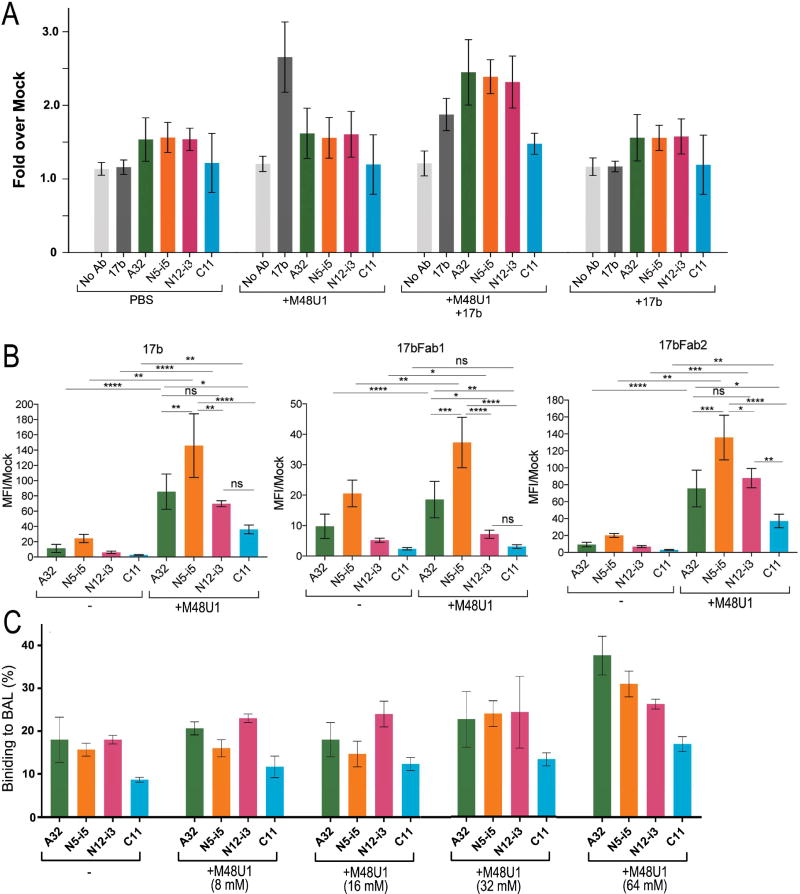
Figure 5. Exposure of A32-like and C11-like epitopes at the surface of HIV-1 infected cells and HIV-1 virions.
(A) CEM.NKr cells were infected with the infectious HIV-1NL-4.3 ADA/GFP molecular clone; 48h post-infection cells were stained with Alexa Fluor 647-conjugated mAbs: 17b, A32, N5-i5, N12-i3 and C11 alone, in the presence of the CD4 mimetic M48U1 (100nM) or together with M48U1 and co-receptor binding site 17b (5µg/ml). Data are the averages of four independent experiments. (B) Binding to HIV-1JR-FL Env trimers expressed at the surface of 293T cells. At 48-hours post-transfection, the exposure of Cluster A epitopes was assessed with Alexa Fluor 647 (AF647)-conjugated A32, N12-i3, or C11 antibodies in the presence or absence of 17b, the 17b Fab-fragment, or the Fab-2 fragment with or without CD4mimetic-M48U1 (100nM). Data are the averages from four independent experiments. Statistical significant was evaluated using paired student t test, * P < 0.05, ** P <0.01, ***P <0.001, **** P < 0.00001; ns, not significant. See also Figure S4 and S5. (C) Effect of M48U1 on the binding of Cluster A mAbs to BaL pseudovirus particles in solution as determined by the FCS assay. The relative fraction of mAb that adopts a lower diffusion coefficient as a result of virion binding is shown. All measurements were performed in triplicate. Average values are shown; error bars indicate standard deviations.