Figure 4. Keap1-mutant cells display a robust sensitivity to glutaminase inhibition.
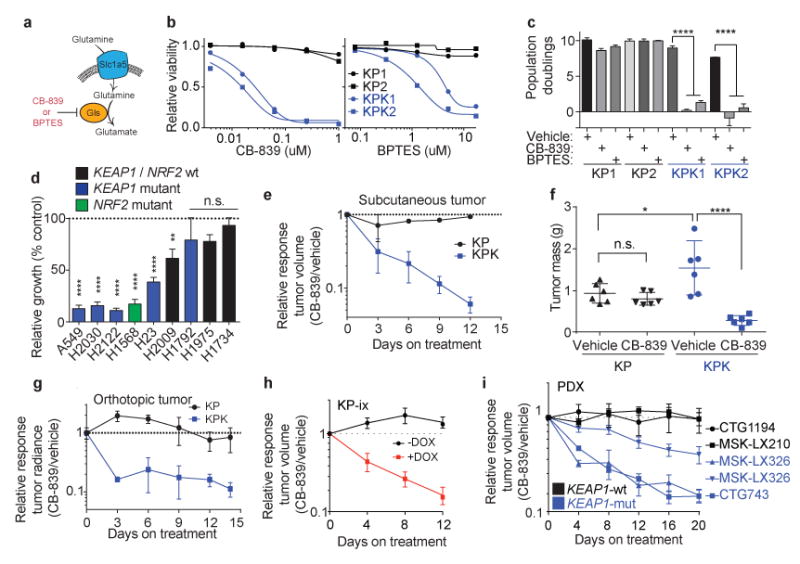
a) Schematic of glutamine uptake by Slc1a5 and hydrolysis of glutamine to glutamate by Gls. Inhibitors of Gls are shown in red. b) Relative viability assayed by cell-titer glo (relative luminescent units) on KP and KPK cells after treatment with CB-839 (left) or BPTES (right) for 72 hrs. All data points are relative to vehicle treated controls (n = 4 technical replicates/data point). c) Cumulative population doublings of KP and KPK cells in the presence of vehicle, CB-839 or BPTES (n = 4 technical replicates/data point) after 6 days in culture. d) Trypan blue exclusion viability counts of indicated human lung cancer cell lines. Each cell line was cultured in the presence of vehicle or 500nM CB-839 (n = 4 technical replicates/cell line). Displayed results are normalized against vehicle treated cell lines after 72 hrs of treatment. A549 and H1975 are TP53-wild type, all others are TP53-mutant. e) Subcutaneous tumor volumes of KP and KPK treated with vehicle or CB-839 starting from day 13 measured over time for 25 days (n = 6 tumors/genotype/treatment). Related to Fig 4f. f) Final tumor masses related to Supplementary Data Fig 11b. *p < 0.05, ****p < 0.0001 obtained from 1-way ANOVA with Tukey's post hoc test. g) Orthotopic growth measurements of KP and KPK cells treated with vehicle or CB-839 starting from day 13 (n = 4 mice/genotype/treatment). Quantitation of luminescence (photon flux) in mice orthotopically transplanted with KP or KPK cells transduced with a vector expressing Luciferase. Relative photon flux calculated by normalizing all time points per animal to initial measurements at 10 days post transplantation. Individual groups depicted in Supplementary Data Fig 11c. ***p < 0.001 obtained from 2-way ANOVA. h) Subcutaneous tumor volumes of KP-ix (inducible GOF-Nrf2) treated with vehicle or CB-839 in the presence or absence of doxycycline (DOX) (n = 6 mice/DOX treatment). Individual groups and full experiment depicted in Supplementary Data Fig 11d. i) Five patient-derived xenograft (PDX) models treated with vehicle or CB-839 for the indicated amount of days. Individual groups and full experiments depicted in Supplementary Data Fig 11g and h. All error bars depict s.e.m.
