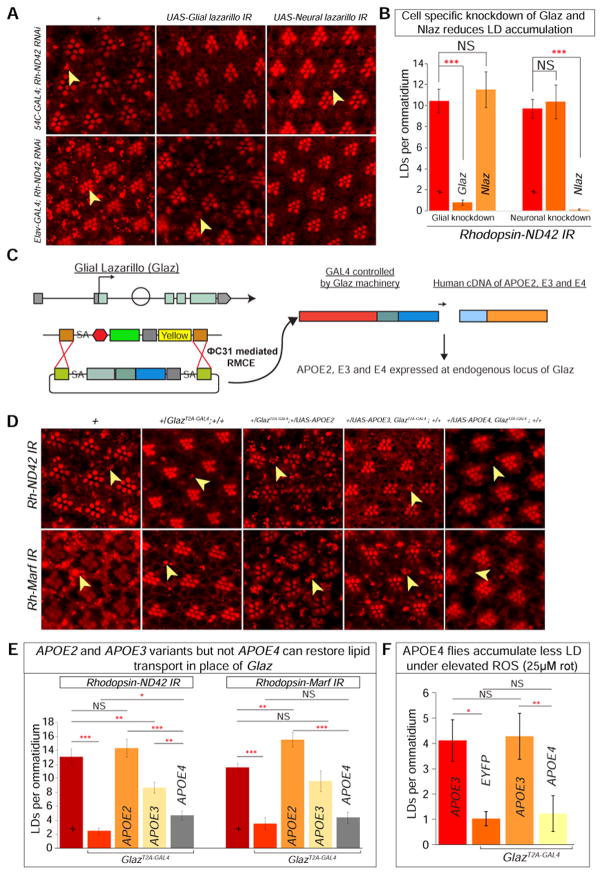
Figure 6. Human APOE4 cannot functionally replace Glaz in lipid transport.
A) a–b. Nile Red stain reveals a baseline of elevated LD accumulation. c–d. Glial knockdown of Glaz in the Rh-ND42 IR background leads to decreased in glial LD accumulation but neuronal knockdown does not alter LD accumulation. e–f. Glial knockdown of Nlaz in the Rh-ND42 IR background does not alter LD accumulation but neuronal knockdown leads to a decrease in glial LD accumulation. B) Quantification of A. (Student’s t-tests, n > 10 animals each). C) MiMIC insertion in the first coding intron of Glaz allows for recombination mediated cassette exchange to insert a Trojan exon containing a splice acceptor (SA), linker, and T2A sequence and a GAL4 sequence followed by a poly A tail. The insertion of the Trojan exon produces flies with a truncated Glaz protein (mutant) and GAL4 which is used to express any UAS-gene. D) a–b. Rh-ND42 IR and Rh-Marf IR exhibit glial LD accumulation. c–d. Removing one copy of Glaz by introducing GlazT2A-GAL4 decreases glial LD accumulation in the Rh-ND42 IR and Rh-Marf IR background. e–f. Replacing the one-copy-loss of Glaz with expression of APOE2 variant restores glial LD accumulation. g–h. Substituting one-copy-loss of Glaz with expression of APOE3 variant restores glial LD accumulation. i–j. Substituting one-copy-loss of Glaz with APOE4 variant does not restore LD accumulation. E) Quantification of D. F) Flies with APOE4 expression in place of Glaz accumulate less LD compared to APOE3 expressing flies when fed rotenone (Kruskal-Wallis test followed by Dunn’s test for significance. n > 10 animals each). Data are represented as mean ± SEM. (*P<0.05, **P<0.005, ***P<0.0005.).