Abstract
Research becomes very significant and meaningful when it addresses a significant public health problem of a region. Fungal keratitis is a serious problem affecting the agrarian poor and hence requires attention from public health specialists. The approach to a public health issue should focus not only on treatment but also prevention or at least show a significant thrust to reduce the morbidity of the problem. At our institution, we have developed a special interest in fungal keratitis and tried to study it in a multitude of aspects. As we put the pieces of the puzzle together, we believe that interest will be rekindled among policymakers, clinicians, microbiologists, pharmaceutical industry, and basic scientists to work together to join forces and take up an integrative approach to managing this problem. It is also believed that the article underscores the need and importance of having a focused approach to ensuring a successful career in clinical research.
Keywords: Drug trials, fungal keratitis, ocular microbiology, research
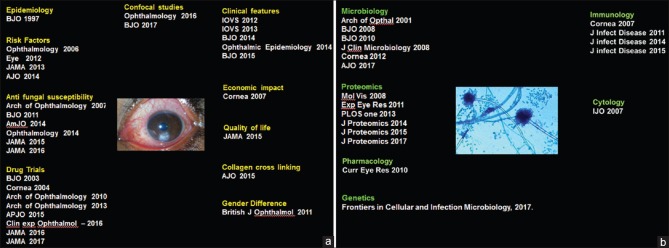
This article is intended to share our experiences in conducting research activities in the field of fungal keratitis at Aravind Eye Care System over the past two decades. Although our research publications include clinical [Fig. 1a] and basic aspects [Fig. 1b], this article selectively deals with our experience in doing clinical research in this field. It does not profess to be a comprehensive review article. There may be certain statements in this article, which the reader may not agree with. This feeling is perfectly understandable, and we would like to respect the sentiments of the reader. At the same time, we hope that this article encourages the reader to take steps to formulate additional research which might end up either supporting or refuting our findings. Ability to appreciate and celebrate our differences with an open mind is a recommended habit which would be very useful for the future researcher on the long road toward the pursuit of true knowledge.
Figure 1.
(a) A compilation of the clinical articles published in the field of fungal keratitis by the authors, (b) a compilation of the basic research articles published in the field of fungal keratitis by the authors
This journey started off with faltering steps and has cruised along with the help of competent professional colleagues, our ability to comprehend things more holistically as we mature (with age and experience), our quest to do something for the hapless patients affected by this condition and also our readiness to accept change. Of course, there is no denying the fact that serendipity played its part by bringing like-minded people together at appropriate time intervals and then like a web, expansionist activities started to get built around the theme of fungal keratitis.
A chance meeting between Dr. Srinivasan and Dr. Gilbert Smolin and Dr. Jack Whitcher at a conference in 1984 sparked off interest in collaborating in the field of corneal infections between Aravind Eye Hospital and Proctor Foundation USA. It would take another 7 years of trust building through exchange of correspondence (in the pre-email era!) before potential interest could fructify into reality. The professional partnership between these two WHO collaborating centres has flourished for more than a quarter century now spanning three generations of ophthalmologists from both institutions. Multiple projects have been conceptualized and executed, resulting in more than 100 publications in high-impact professional journals. This enriching process stands as a testimony to the power of international high quality, high impact collaboration between two like-minded institutions in addressing the social needs of the population.
This article is a synopsis of some of the important clinical studies done by us in the field of fungal keratitis.
Fungal Keratitis is a Big Public Health Problem
During the nineties, it was evident that, from an institutional perspective, we were seeing a lot of patients with corneal ulceration at Aravind. However, no population-based data existed in the region, or for that matter in India at that point of time. In 1996, investigators from Aravind along with the collaborators at the Proctor foundation performed a study to find out the incidence of corneal ulceration in Madurai district, Tamil Nadu.[1] This retrospective population-based study surveyed all corneal ulcers occurring in 1993 in Madurai district with a population of around 35 lakhs. There were 1148 cases of corneal ulceration recorded in medical records in the district, yielding an annual incidence of 3.4 cases of corneal ulceration per 10,000 population. However, by carefully questioning all of the eye care practitioners in the district and examining patient records, our investigators were able to extrapolate a much higher (and truer) estimated annual incidence of 11.3/10,000 population. To this date, this figure is constantly used by investigators in India, when they refer to the incidence of corneal ulceration in the country.
So, how do these numbers stack up when compared to other countries? The incidence of corneal ulceration in the United States from 1980 to 1988 was reported to be 11/100,000, implying that the incidence of corneal ulceration in Madurai district was ten times higher. Applying the incidence rate derived from our study, it was estimated that around 50,000 new ulcers develop in the state of Tamil Nadu annually. If extrapolated to the whole of India, this number swells up to a staggering 8, 40,000 people. This magnitude prompted the authors to write a special commentary in the British Journal of Ophthalmology, terming the corneal ulceration in India to be a silent epidemic.[2]
Having established the magnitude of the problem, we then performed a prospective study to consider epidemiology and etiological diagnosis of corneal ulceration in the region. This was done on the 434 patients diagnosed with a central corneal ulcer at Aravind Eye Hospital, Madurai between January 1 and March 31, 1994. All these patients underwent standard microbiological investigations including smear and culture. Corneal cultures were positive in 297 of these patients (68.4%). Of these individuals with positive cultures, 140 (47.1%) had pure bacterial infections, 139 (46.8%) had pure fungal infections, 15 (5.1%) had mixed bacteria and fungi, and three (1%) grew pure cultures of Acanthamoeba. Fusarium (47%) was the most common fungal pathogen isolated followed by Aspergillus species. The most common bacteria isolated was Streptococcus pneumoniae (44%) followed by Pseudomonas (14%).
Even though anecdotally it was felt that fungal ulcers were becoming common, this study established the magnitude of the fungal etiology.[3] In recent times, it is felt that fungi are becoming increasingly more and more common as a culprit in causing keratitis. To get a firm idea, we analyzed the trends in bacterial and fungal keratitis over a 10 years’ period between 2002 and 2012.[4] Of the 23,897 corneal ulcer patients who had their corneal smear examined during this period, a fungal pathogen organism was identified in 34.3%, a bacterial organism in 24.7% and no organism in 38.3%. During the period, the annual number of keratitis cases due to bacteria decreased from 677 to 412, and the annual number due to fungus increased from 609 to 863, thus confirming our hypothesis. In analyses accounting for the total number of outpatients seen each year, the decline in a number of smear-positive for bacteria was statistically significant (P < 0.001), but the incidence in the number positive for fungus was not (P = 0.73).
We then wanted to see whether the epidemiology was any different in the pediatric population. Previous studies had shown that bacteria were the most common cause of infectious keratitis in children. Pseudomonas and Staphylococcus epidermidis were the commonly isolated bacteria in children with infective keratitis. Our study was a retrospective study with the plan being to estimate the risk factors, microbiological profile, and clinical outcomes of infectious keratitis affecting pediatric population.[5] This study included 240 eyes of 234 children. The cultures were positive in 142 (74.3%) eyes. Fungi was the most common infectious agent isolated in culture (54.2%), followed by bacteria (40.8%) and Acanthamoeba. Contrary to previous reports, fungi were the most common etiological organism in the causation of infectious keratitis in children in our study population. Fusarium was the most common fungal species isolated. This data were similar to that obtained from adult patients with infectious keratitis in the region. This data are extremely valuable, since it may be a common practice in primary and secondary centres to start empirical treatment in young children since they may not be cooperative for microbiological investigations. In such instances, the findings from our study need to be kept in mind while initiating therapy.
What are the Risk Factors?
Different risk factors have been ascribed to fungal keratitis. Broadly, the yeasts are thought to be associated with systemic immunosuppression while filamentous fungi often are associated with persons involved in agrarian activities. In one of our earlier retrospective study, we found that in patients with fungal keratitis treated with Natamycin 5% monotherapy, large ulcer size and infection with Aspergillus were predictors of a poor outcome.[6] That the larger ulcers leading to a poorer outcome was a no-brainer but the second finding was interesting. Historically, it was believed that Fusarium was a virulent organism. In fact, in his classic article on the principles of management of oculomycosis, Jones reported that Fusarium solani was far more destructive than Aspergillus.[7]
Immediately, many questions come to mind: Did something happen during these 40 years that Aspergillus became more virulent than Fusarium? Does the virulence of these fungal pathogens differ in different geographic regions? These are critical questions to ponder about, especially, in the context of Aspergillus being the most common etiological organism amongst the Northern and Eastern regions of India, while Fusarium is reported to be the most common organism in the Western and the Southern part of the country. In our mind, the concept of considering that each genera of fungi having a specific virulence pattern is overly simplistic.
Five years later, we performed a prospective study to see whether the risk factors have changed.[8] This study also confirmed that a larger infiltration size leads to a poorer visual outcome. Older age and male gender were more associated with poor vision. This study threw up an interesting observation that pigmentation of a corneal ulcer can be a prognostic factor for poor visual outcomes. This was interesting since earlier studies had hypothesized that pigmentation induces low virulence and less severe inflammation.[9] These variations in findings give ample scope for future researchers to pursue these areas and design even more robust studies to establish the veracity of these studies.
It Is an Expensive Disease
Corneal ulcers are a devastating economic problem for patients and their families. Despite the high-cost implications, they do not attract as much attention as other ocular conditions including cataracts, refractive errors, and childhood blindness. The relatively young age of the patient, the disproportionately poor socioeconomic background from where they come from and the associated loss of man years of economic productivity takes a big economic toll. Added to this is the important factor, that even in the best of the scenario of the ulcer healing, the visual rehabilitation is not optimal.
In a bid to estimate the costs of treating corneal ulcers at a tertiary eye care center from a patient's perspective, we performed a prospective cohort study in 2006 involving around 498 patients accessing the center in a defined period.[10] The mean duration of the onset of symptoms before presentation at the cornea service was 13.1 ± 19.9 days. The mean follow-up duration was 34.8 ± 28.2 days. The mean total cost to diagnose and appropriately treat a case of keratitis such that the patient had vision better than 6/18 or better in the final follow-up was around 4000 rupees. The costs to the patient to receive appropriate care for corneal ulcers in this population was higher than the average monthly wage for this group of individuals at that point of time. It has to be kept in mind that at least a third of the total patients would have ended up with impaired vision. In fact, there is a mismatch between the patient and the ophthalmologist on the concept of “success,” while treating a patient of corneal ulcer. Hence, it is very important to concentrate on a preventive strategy to combat corneal infections.
Importance of Ocular Microbiology
Even though we are often taught the characteristic descriptions ascribed to fungal and bacterial ulcers, it is often difficult to diagnose them in real life without the help of microbiology. In the initial stages of corneal ulceration, the ulcer morphology may have these distinct characteristics, but things become confusing when the ulcer increases in size and intensity [Fig. 2]. Unfortunately, the patients present to the clinicians in a fairly late stage. We undertook a study to determine whether established cornea specialists could predict the organism based on the clinical manifestation of the corneal ulcer.[11] Eighty photographs of eyes with culture-proven bacterial keratitis or smear proven fungal keratitis were randomly selected from two clinical trials - The mycotic ulcer treatment trial (MUTT) and the steroids for corneal ulcer trial (SCUT), both of which were undertaken at Aravind. Fifteen cornea specialists from the Proctor Foundation, USA and the Aravind Eye care system assessed the photographs and were asked to predict the most likely causative organism. The cornea specialists were able to correctly distinguish bacterial from fungal etiology in only 66% of the time (P < 0.001). Even though, they were able to do this slightly better than chance, it was very clear, that with all their clinical experience, they could not be very certain about the organism. More specific categorization led to even poorer clinical distinction. The Gram stain, genus and species were accurately predicted 46%, 25%, and 10% of the time, respectively. However, the saving grace was that the presence of an irregular feathery border was very clearly identified as that caused by a fungus [Fig. 3]. Although certain clinical signs of infectious keratitis may be associated with a bacterial or fungal etiology, this study highlights the importance of obtaining appropriate microbiological testing during the initial clinical examination. Both KOH [Fig. 4] and Grams stain [Fig. 5] are easy to do and identify the fungi. This importance may be more relevant in the Indian context, where fungi and bacteria cause corneal ulcers almost in equal proportions and empirical regimen is not a recommended mode of initiating treatment.
Figure 2.
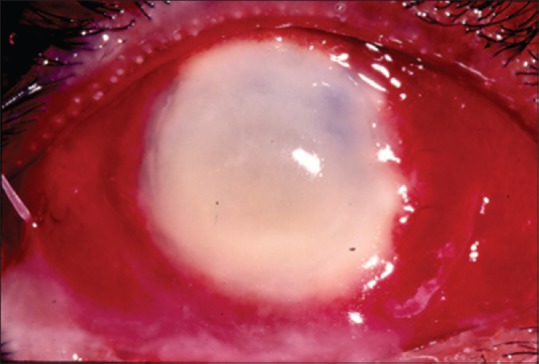
Advanced corneal ulcer. Clinical examination alone will not be enough to make the diagnosis
Figure 3.
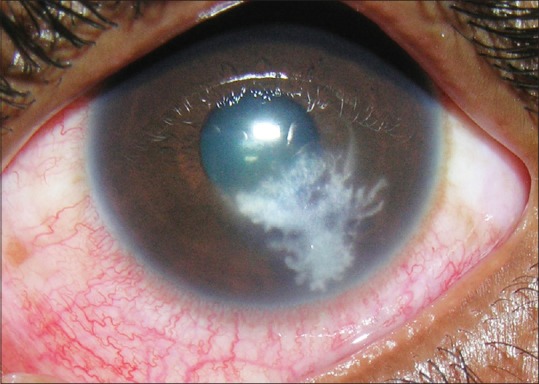
A typical fungal ulcer with feathery margins
Figure 4.

KOH wet mount with fungal hyphae
Figure 5.
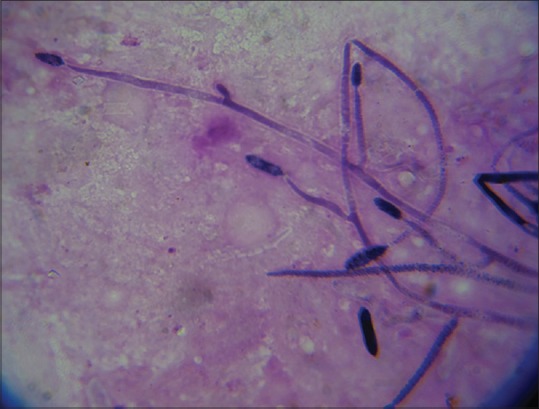
Grams stain with fungal hyphae
Clinical Outcomes
Fungal keratitis is known to be a more prolonged and severe disease than bacterial keratitis. Our department conducted two large well-designed prospective clinical trials, namely, the SCUT for patients with bacterial keratitis and MUTT for patients with fungal keratitis. This unique scenario enabled us to compare clinical outcomes in ulcers due to bacteria and fungus using data collected from two similarly structured prospective trials. When the data were compared between the two studies, it was found that fungal keratitis had nearly five times as many corneal perforations, and longer healing times.[12] While there are inherent challenges in combining data from multiple clinical studies these trials were a special case, in that the trials were conducted concurrently by the same investigators, outcomes were measured according to identical protocols and the inclusion and exclusion criteria were nearly identical for both trials.
Confocal Microscopy
Recently, confocal microscopy has proved to be very useful as an adjuvant noninvasive tool to aid in the diagnosis of microbial keratitis [Fig. 6]. We performed a study to determine the diagnostic accuracy of in vivo confocal microscopy (IVCM) using Heidelberg retinal tomograph 3 for moderate-to-severe microbial keratitis.[13] It was a double-masked prospective cohort study. All consecutive moderate to severe corneal ulcers were scanned by the laser scanning IVCM. The study was performed by five graders who were masked to clinical features and microbiology. The main outcome measures were sensitivity, specificity, and positive and negative predictive values of IVCM compared with those of a reference standard of positive culture. Out of the 239 patients enrolled, fungi infection was detected in 176 (74%) by microbiological methods. IVCM had an overall pooled (5 graders) sensitivity of 85.7% and pooled specificity of 81.4% for fungal filament detection. The agreement between the graders was good for definite fungus (k 0.88–0.95) and so was their repeatability (k 0.88–0.95). We concluded that laser scanning IVCM performed with experienced confocal graders has high sensitivity and test reproducibility for detecting fungal filaments. This imaging modality was particularly useful for detecting organisms in deep ulcers where culture and light microscopy results were inconclusive.
Figure 6.
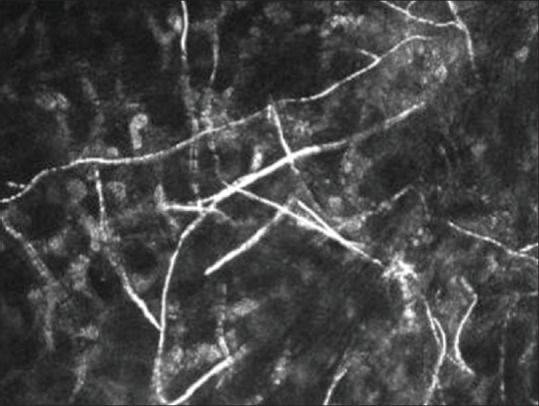
In vivo confocal microscopy showing fungal hyphae with different branching patterns
Our next step was to see whether the confocal microscopy could distinguish different fungal species with confidence. This was important since we had shown that the clinical outcomes in fungal keratitis vary between Fusarium and Aspergillus and moreover, these two different genera respond differentially to different antifungal drugs. Previous studies had postulated that the branching patterns of fungi as seen in IVCM images of keratitis could be used to differentiate fungal species.[14] In addition to the branching pattern, we also looked for other features such as sporulation along filaments (adventitious sporulation) and dichotomous branching.
In our study of 68 patients of Fusarium keratitis and 30 patients of Aspergillus keratitis, it was found that the mean branch angle for Fusarium species was 59.7° (95% confidence interval [CI], 57.7°–61.8°) and for Aspergillus species was 63.3° (95% CI, 60.8°–65.8°) P = 0.07. No adventitious sporulation was detected in Fusarium species ulcers. Dichotomous branching was also not unique and was seen in seven cases of Aspergillus keratitis and 4 cases of Fusarium keratitis. There was very little difference in the branching angle of Fusarium and Aspergillus species.[15] We concluded that although IVCM remains a valuable tool to detect fungal filaments in fungal keratitis, it cannot be used to distinguish Fusarium from Aspergillus species and culture remains essential to determine the fungal species.
Drug Trials
For a disease of such magnitude and a potential to cause profound morbidity, research publications in the area of drug trials in fungal keratitis are far and few in between. In fact, we published the first-ever randomized control trial in the field of fungal keratitis as late as 2003.[16] Two years before this, Aurolab had launched a new antifungal formulation in India, namely, Econazole. This drug was very popular in the United Kingdom and was widely used especially in Moorfields. Interestingly, two earlier case studies from India had talked about the efficacy of this drug.[17,18] We randomized 116 patients to receive either Econazole or Natamycin. The endpoint was kept at 4 weeks. This study concluded that Econazole and Natamycin were comparable in the treatment of filamentary fungal keratitis.
While we were doing this clinical trial, a thought struck us whether the concurrent use of both these drugs would prove additive. The rationale behind this thought was that Natamycin and Econazole have two different modes of action. While Natamycin binds preferentially to ergosterol on the fungal plasma membrane and causes localized membrane disruptions by altering membrane permeability, Econazole exhibits antifungal activity by inhibiting fungal cell membrane synthesis. With this thought in mind, we designed a prospective study to see for the efficacy of using this combination study. This study, however, was not a randomized study and was performed on 47 subjects who had concurrent use of both 1% Econazole and 5% Natamycin. This was compared with a historical control from our previous study performed using a similar protocol. Data from this study did not suggest any additional benefits or deleterious effects with the concurrent use of 5% Natamycin and 2% Econazole as topical applications for fungal keratitis.[19]
At around this time, isolated case reports on the benefits of Voriconazole started appearing in the world literature. In vitro susceptibility studies performed in our microbiology laboratory revealed a superior profile for Voriconazole,[20] and this finding again was reiterated in another of our studies 2 years later as well.[21] At this juncture, we decided to perform a survey across a section of international cornea specialists for their choice of preference of antifungals. According to this survey, 80% of the corneal specialists believed that existing treatments were only moderately effective and that, if available, Voriconazole would be the preferred treatment of choice for fungal keratitis.[22] This was understandable since a new antifungal drug was being introduced after a long time and which also had a good anecdotal backup both in the clinical reports as well as antifungal susceptibility data. We decided then to perform a randomized clinical trial to compare the efficacy of the new drug (Voriconazole) with that of the existing gold standard (Natamycin) in the treatment of fungal keratitis.
One of the things, we learnt early on in our career in ophthalmic research is to perform pilot or exploratory studies around our hypothesis. These pilot studies expose the teething difficulties and fallacies in our hypothesis and can be used to modify and refine the protocol before embarking on the main study. Interestingly we chose to consider visual acuity as the most important outcome for this study, which was a novel idea since almost all previous studies (including our previous trials) had considered epithelial healing and nonprogression of stromal infiltration as the primary endpoint. We did not think that the epithelial healing was the optimal primary outcome for this study because one of our interventions was epithelial debridement and also because the epithelium can heal despite an active underlying corneal infiltrate in fungal keratitis. Our pilot study was performed on 120 patients who were randomized to receive either topical Voriconazole or Natamycin. Since anecdotal reports (personal communication) suggested that clinicians believe that a therapeutic re-scraping would help in the treatment of fungal keratitis, we randomized a part of this study group to undergo a repeat therapeutic scraping at 1 week. This study concluded that there were no significant differences in the visual acuity, scar size, and perforations between Voriconazole- and Natamycin-treated patients. There was a trend toward a 2 line benefit with Voriconazole treatment, but it was not superior to Natamycin. Very interestingly, corneal scraping was associated with worse best-corrected visual acuity at 3 months after adjusting for the drug (P = 0.06). While we still believe that a debridement is effective in a plaque-like lesion (where we can easily lift it off, especially in a pigmented lesion), there is no merit in deploying this strategy for all fungal ulcers.
This pilot study itself took around 2 years and gave us a good idea about the feasibility of the study. Results from this pilot study also allowed us to calculate the sample size and it was decided to enroll 365 patients.
The study was named as MUTT. We had to screen 940 patients ultimately to get the desired number. This study had a lot of inherent advantages in avoiding bias. It was a study funded by the National Eye Institute, USA. Alcon donated Natamycin and Pfizer donated Voriconazole for the study. The study participants were kept in house in the hospital for a minimum period of a week to ensure treatment compliance. This study was also monitored by an expert international data and safety monitoring committee (DSMC) which reviewed the data at regular intervals. The study was terminated at 323 patients on the recommendation of the DSMC since the analysis of the interim data was very conclusively in favor of one arm of the trial. In fact, this was the first clinical trial, in which we were involved, which was stopped much before the planned recruitment, since the results were conclusive. The MUTT concluded that Natamycin treatment was associated with significantly better clinical and microbiological outcomes than Voriconazole treatment for smear-positive filamentous fungal keratitis with much of the differences attributable to improved results in Fusarium keratitis. This was also the first time, where the differential sensitivity of the drugs to different fungus genus was reported. It was a well-known fact that the susceptibility profile of the Gram positive and Gram negative organisms were different and MUTT also brought out a similar profile of differential sensitivity among fungi. MUTT also recommended that Voriconazole should not be used as monotherapy in filamentous keratitis.
The success of any research comes when it is right, rigorous, responsible, reliable, and more importantly repeatable. In the MUTT study, the Voriconazole used was reconstituted from a systemic preparation, since no topical formulation was available. Meanwhile, Aurolab had manufactured an ophthalmic preparation of Voriconazole and investigators at LV Prasad institute performed a similar study using the commercial eye drop preparation of Voriconazole.[23] The title of the study was aptly named as reappraisal of topical 1% Voriconazole and 5% Natamycin in the treatment of fungal keratitis in a randomized trial. This study also conclusively reiterated the findings of the two previous MUTT studies and concluded that Natamycin was superior to Voriconazole.
The MUTT studies had international ramifications. The treatment protocol at the world-renowned Moorfields center was altered and they commented in a published article that “More recently we changed from Voriconazole 1% to Natamycin 5% (in the treatment of fungal keratitis). This change was informed by the results of the MUTT 1 trial from South India, which found Natamycin 5% to be superior to Voriconazole 1%, particularly for the treatment of Fusarium.”[24] Investigators in Wills Eye Hospital in Philadelphia also reiterated that their treatment protocol was changed after the MUTT results were published. We were delighted that our study could influence treatment protocols across the globe and especially in well-renowned centres such as Moorfields and Wills. This was even more important in the context that our study did not support the hitherto held view amongst the cornea specialists across the world that Voriconazole was superior.
It has been a common practice to use oral antifungal therapy in addition to topical antifungal drugs in patients with severe and deep fungal keratitis. We had earlier published a study which revealed no added benefit in supplementing oral ketoconazole to topical antifungals in fungal keratitis.[25] Since systemic Voriconazole is being widely used for systemic aspergillosis, we decided to perform a randomized clinical trial using oral Voriconazole supplementation to arrive at a definitive conclusion. This prospective study, termed MUTT II, was performed in India and Nepal and compared oral Voriconazole with placebo in addition to topical antifungals in the treatment of severe fungal keratitis. A total of 2133 patients with smear positive ulcers were screened to first arrive at 787 eligible patients. Out of these, 240 patients fitted into the inclusion and exclusion criteria of the study. The study participants were then randomized to receive either oral Voriconazole (400 mg administered twice daily for 24 h followed by a maintenance dose of 200 mg twice daily for 20 days) or oral placebo. Both groups received topical Natamycin and topical Voriconazole concurrently. The primary outcome of the trial was the rate of corneal perforation or the need for therapeutic keratoplasty within 3 months. There were a total of 65 perforations with 30 (46.2%) occurring in the placebo arm and 35 (53.8%) in the oral Voriconazole arm. In this study, we did not find any difference in the rate of corneal perforation or the need for TPK between the groups (hazard ratio 0.82, 95% CI, 0.57–1.18, P = 0.29). In addition, patients receiving Voriconazole experienced a total of 58 adverse events (48.7%) compared with 28 adverse events in the placebo group. We concluded that there was no benefit in adding oral Voriconazole to topical antifungal agents in the treatment of severe fungal keratitis.[26]
The availability of ancillary data obtained through the conduct of a robust clinical trial provided additional information. We analyzed the minimum inhibitory concentration (MIC) data for both Voriconazole and Natamycin. While the mean Natamycin MIC remained unchanged, the mean Voriconazole MICs for all organisms increased from 1.86 μg/ml in 2010 to 3.79 μg/ml in 2011. In essence, we found an increase in azole resistance of filamentous fungi recovered from baseline corneal cultures during MUTT 1 after controlling for the infectious organism.[27] This assumes significance because azole antifungals are some of the most common antifungals used in agriculture, which may have promoted increasing resistance among the fungal species. It is of special interest to note that Natamycin is not used in agriculture.
Collagen Cross-Linking
After collagen cross-linking (CXL) became popular for keratoconus, multiple reports started appearing in the world literature citing its efficacy to be used for infectious keratitis. All of them were either case reports or case series. We performed a randomized clinical trial to assess the efficacy of CXL as an adjuvant to appropriate antifungal therapy in non-resolving deep stromal fungal keratitis.[28] The eyes with culture-positive deep stromal fungal keratitis not responding to appropriate medical therapy for 2 weeks were randomized to receive either adjuvant CXL or no adjuvant treatment. Antifungal medical therapy was continued in both groups. The prespecified primary outcome was treatment failure at 6 weeks after enrolment, defined as perforation and/or increase I ulcer size >2 mm. The trial was stopped before enrolment because of a marked difference in the rate of perforation between the two groups. Of the 13 cases enrolled in the study, 6 were randomized to the CXL group and 7 to the nonCXL group. The CXL group experienced more perforations than the nonCXL group (4 vs. zero) P = 0.02 [Fig. 7a and b]. Five eyes in the CXL group and 3 eyes in the nonCXL group experienced treatment failure by 6 weeks. We concluded that CXL used as adjuvant therapy does not aid in the treatment of fungal keratitis.
Figure 7.
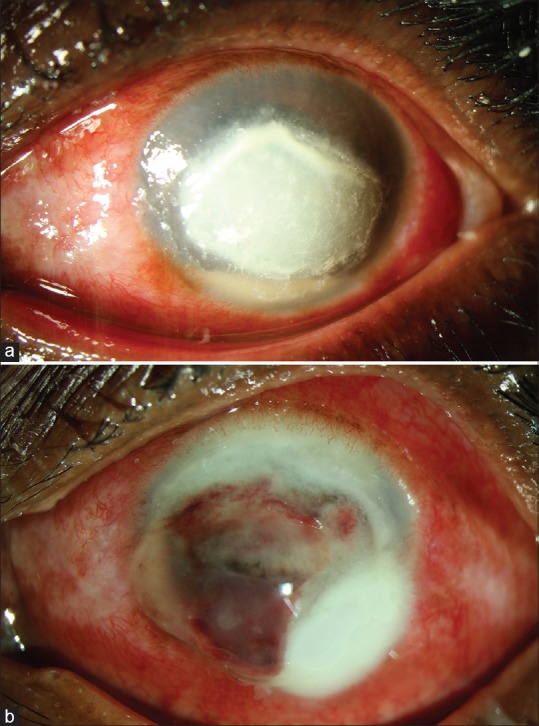
(a) Fungal corneal ulcer before collagen cross-linking with ultraviolet-A and riboflavin, (b) fungal corneal ulcer perforation after collagen cross-linking with ultraviolet-A and riboflavin
Conclusion
As we continue to do our research, we are very aware that the last word has not been said about this condition. A seemingly small ulcer relentlessly progresses even in spite of adequate treatment [Fig. 8a–c]. We are painfully aware of the fact that we are not treating the disease holistically-rather, we are just concentrating on killing the fungus. The exuberant and the intemperate inflammatory response of the host may be a determining factor in ultimately deciding about the success of our intervention. Unfortunately, at this point of time, none of our treatment regimens are tailored to address this phenomenon. The future generations of ophthalmologists would wonder why we did not think about this aspect and may probably consider our current treatment strategies as primitive. The ophthalmologists will evolve, but so will the fungus. Both groups will mature with time, and both of them will continue to plan strategies to overcome each other. While some treatment procedures may be revolutionary, it is the constant evolutionary ideas which have seen broad-based advancements in the medical field. This is the inevitable path of scientific progress, and we look forward in planning and executing more thought processes into this field of fungal keratitis.
Figure 8.
(a) A moderate ulcer on presentation, (b) the same ulcer progressing in spite of antifungal medication, (c) the entire cornea eaten up with a resultant pseudocornea
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gonzales CA, Srinivasan M, Whitcher JP, Smolin G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol. 1996;3:159–66. doi: 10.3109/09286589609080122. [DOI] [PubMed] [Google Scholar]
- 2.Whitcher JP, Srinivasan M. Corneal ulceration in the developing world – A silent epidemic. Br J Ophthalmol. 1997;81:622–3. doi: 10.1136/bjo.81.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srinivasan M, Gonzales CA, George C, Cevallos V, Mascarenhas JM, Asokan B, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–71. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lalitha P, Prajna NV, Manoharan G, Srinivasan M, Mascarenhas J, Das M, et al. Trends in bacterial and fungal keratitis in South India, 2002-2012. Br J Ophthalmol. 2015;99:192–4. doi: 10.1136/bjophthalmol-2014-305000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aruljyothi L, Radhakrishnan N, Prajna VN, Lalitha P. Clinical and microbiological study of paediatric infectious keratitis in South India: A 3-year study (2011-2013) Br J Ophthalmol. 2016;100:1719–23. doi: 10.1136/bjophthalmol-2015-307631. [DOI] [PubMed] [Google Scholar]
- 6.Lalitha P, Prajna NV, Kabra A, Mahadevan K, Srinivasan M. Risk factors for treatment outcome in fungal keratitis. Ophthalmology. 2006;113:526–30. doi: 10.1016/j.ophtha.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 7.Jones BR. Principles in the management of oculomycosis. XXXI Edward Jackson memorial lecture. Am J Ophthalmol. 1975;79:719–51. doi: 10.1016/0002-9394(75)90730-8. [DOI] [PubMed] [Google Scholar]
- 8.Prajna NV, Krishnan T, Mascarenhas J, Srinivasan M, Oldenburg CE, Toutain-Kidd CM, et al. Predictors of outcome in fungal keratitis. Eye (Lond) 2012;26:1226–31. doi: 10.1038/eye.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg P, Gopinathan U, Choudhary K, Rao GN. Keratomycosis: Clinical and microbiologic experience with dematiaceous fungi. Ophthalmology. 2000;107:574–80. doi: 10.1016/s0161-6420(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 10.Prajna VN, Nirmalan PK, Saravanan S, Srinivasan M. Economic analysis of corneal ulcers in South India. Cornea. 2007;26:119–22. doi: 10.1097/ICO.0b013e31802b36dc. [DOI] [PubMed] [Google Scholar]
- 11.Dalmon C, Porco TC, Lietman TM, Prajna NV, Prajna L, Das MR, et al. The clinical differentiation of bacterial and fungal keratitis: A photographic survey. Invest Ophthalmol Vis Sci. 2012;53:1787–91. doi: 10.1167/iovs.11-8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prajna NV, Srinivasan M, Lalitha P, Krishnan T, Rajaraman R, Ravindran M, et al. Differences in clinical outcomes in keratitis due to fungus and bacteria. JAMA Ophthalmol. 2013;131:1088–9. doi: 10.1001/jamaophthalmol.2013.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chidambaram JD, Prajna NV, Larke NL, Palepu S, Lanjewar S, Shah M, et al. Prospective study of the diagnostic accuracy of the in vivo laser scanning confocal microscope for severe microbial keratitis. Ophthalmology. 2016;123:2285–93. doi: 10.1016/j.ophtha.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brasnu E, Bourcier T, Dupas B, Degorge S, Rodallec T, Laroche L, et al. In vivo confocal microscopy in fungal keratitis. Br J Ophthalmol. 2007;91:588–91. doi: 10.1136/bjo.2006.107243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chidambaram JD, Prajna NV, Larke N, Macleod D, Srikanthi P, Lanjewar S, et al. In vivo confocal microscopy appearance of Fusarium and Aspergillus species in fungal keratitis. Br J Ophthalmol. 2017;101:1119–23. doi: 10.1136/bjophthalmol-2016-309656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prajna NV, John RK, Nirmalan PK, Lalitha P, Srinivasan M. A randomised clinical trial comparing 2% econazole and 5% natamycin for the treatment of fungal keratitis. Br J Ophthalmol. 2003;87:1235–7. doi: 10.1136/bjo.87.10.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arora I, Kulshrestha OP, Upadhaya S. Treatment of fungal corneal ulcers with econazole. Indian J Ophthalmol. 1983;31(Suppl 1):1019–21. [PubMed] [Google Scholar]
- 18.Mahashabde S, Nahata MC, Shrivastava U. A comparative study of anti-fungal drugs in mycotic corneal ulcer. Indian J Ophthalmol. 1987;35:149–52. [PubMed] [Google Scholar]
- 19.Prajna NV, Nirmalan PK, Mahalakshmi R, Lalitha P, Srinivasan M. Concurrent use of 5% natamycin and 2% econazole for the management of fungal keratitis. Cornea. 2004;23:793–6. doi: 10.1097/01.ico.0000134193.64357.82. [DOI] [PubMed] [Google Scholar]
- 20.Lalitha P, Shapiro BL, Srinivasan M, Prajna NV, Acharya NR, Fothergill AW, et al. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch Ophthalmol. 2007;125:789–93. doi: 10.1001/archopht.125.6.789. [DOI] [PubMed] [Google Scholar]
- 21.Lalitha P, Prajna NV, Oldenburg CE, Srinivasan M, Krishnan T, Mascarenhas J, et al. Organism, minimum inhibitory concentration, and outcome in a fungal corneal ulcer clinical trial. Cornea. 2012;31:662–7. doi: 10.1097/ICO.0b013e31823f8ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loh AR, Hong K, Lee S, Mannis M, Acharya NR. Practice patterns in the management of fungal corneal ulcers. Cornea. 2009;28:856–9. doi: 10.1097/ICO.0b013e318199fa77. [DOI] [PubMed] [Google Scholar]
- 23.Sharma S, Das S, Virdi A, Fernandes M, Sahu SK, Kumar Koday N, et al. Re-appraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomised trial. Br J Ophthalmol. 2015;99:1190–5. doi: 10.1136/bjophthalmol-2014-306485. [DOI] [PubMed] [Google Scholar]
- 24.Ong HS, Fung SS, Macleod D, Dart JK, Tuft SJ, Burton MJ, et al. Altered patterns of fungal keratitis at a London ophthalmic referral hospital: An eight-year retrospective observational study. Am J Ophthalmol. 2016;168:227–36. doi: 10.1016/j.ajo.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajaraman R, Bhat P, Vaidee V, Maskibail S, Raghavan A, Sivasubramaniam S, et al. Topical 5% Natamycin with oral ketoconazole in filamentous fungal keratitis: A Randomized controlled trial. Asia Pac J Ophthalmol (Phila) 2015;4:146–50. doi: 10.1097/APO.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 26.Prajna NV, Krishnan T, Rajaraman R, Patel S, Srinivasan M, Das M, et al. Effect of oral voriconazole on fungal keratitis in the mycotic ulcer treatment trial II (MUTT II): A Randomized clinical trial. JAMA Ophthalmol. 2016;134:1365–72. doi: 10.1001/jamaophthalmol.2016.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prajna NV, Lalitha P, Rajaraman R, Krishnan T, Raghavan A, Srinivasan M, et al. Changing azole resistance: A Secondary analysis of the MUTT I randomized clinical trial. JAMA Ophthalmol. 2016;134:693–6. doi: 10.1001/jamaophthalmol.2016.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uddaraju M, Mascarenhas J, Das MR, Radhakrishnan N, Keenan JD, Prajna L, et al. Corneal cross-linking as an adjuvant therapy in the management of recalcitrant deep stromal fungal keratitis: A Randomized trial. Am J Ophthalmol. 2015;160:131–400000. doi: 10.1016/j.ajo.2015.03.024. [DOI] [PubMed] [Google Scholar]