Abstract
Purpose:
To evaluate the efficacy and safety of valproic acid (VPA) treatment in patients with retinitis pigmentosa (RP).
Methods:
A total of 48 eyes of 24 patients (13 males, 11 females) with RP prescribed VPA were included. The length of VPA treatment was 6–12 months (mean 9.4 months). Parameters evaluated were best-corrected visual acuity (BCVA) (logarithm of the minimum angle of resolution [logMAR]), visual field analyses (VFAs) with Humprey automated perimetry, multifocal electroretinography (ERG) with Roland-RETI scan, and VPA side effects.
Results:
Mean age was 34.3 ± 10.3 years (range 18–56 years). Fifteen of the patients (30 eyes) had two ERG and VFA tracings, allowing comparison between baseline and follow-up (range 6–12 months). Mean BCVA before and after VPA therapy was 0.36 ± 0.38 and 0.36 ± 0.37 logMAR, respectively (P = 0.32). Quantitative perimetric indices including mean deviation and pattern standard deviation were not significantly changed after VPA therapy (P > 0.05). P1 amplitudes (in terms of nV/deg2 and mV) of ERG waves were significantly decreased in the rings 1, 3, and 4 after VPA therapy (P < 0.05). Regarding the N1 amplitudes, the only significant decrease was observed in area 1 (P = 0.03). In addition, N1 latency was significantly increased in area 3 after VPA therapy (P = 0.04).
Conclusions:
VPA therapy did not have any significant benefit on BCVA and VFA. In addition, it may be associated with decline in some ERG parameters. Therefore, physicians should avoid prescribing VPA for RP until its safety and efficacy are appropriately evaluated.
Keywords: Electroretinogram, retinitis pigmentosa, valproic acid, visual field
Retinitis pigmentosa (RP) is an inherited retinal dystrophy characterized by nyctalopia and loss of peripheral vision. RP can lead to central vision loss due to progressive degeneration of rod and cone photoreceptor cells.[1]
The disease is heterogeneous genetically, and over 45 genes for RP have been identified.[1] The diverse range of genes responsible for RP has made targeted therapy difficult. Research indicates that nutritional interventions, such as Vitamin A palmitate[2] and docosahexaenoic acid, an omega-3 fatty acid,[3] may slow progression of the disease in some forms of RP, yet benefits from these supplements are modest.
Neuroprotection is another mode of treatment, with several ongoing trials evaluating ciliary neurotrophic growth factor for the treatment of RP.[4] Recently, valproic acid (VPA) has been discussed as a potential treatment for RP. VPA is typically used as an anticonvulsant and mood stabilizer, and it is known to cause gamma-aminobutyric acid (GABA) inhibitory effects in the central nervous system.[5] Collectively, this body of evidence suggests that VPA may be an appropriate therapy for patients with retinal dystrophies due to its inhibitor effect on histone deacetylase[6] and the inflammatory response pathway via apoptosis of microglial cells.[7,8] VPA is also known to downregulate complement proteins and increase the levels of various neurotrophic factors.[9,10] However, VPA has been documented to have a large number of adverse drug reactions, including hepatotoxicity and neurological and mitochondrial toxicity.[11,12]
Its therapeutic benefits on RP are still inconclusive and controversial. In 2011, Clemson et al. published a retrospective study suggesting that RP caused an improvement in visual field (VF) after an average of 4 months of oral VPA treatment with tolerable adverse effects.[13] While the results of this study seem promising, further studies found that VPA appears to be associated with visual acuity (VA) and VF decline as well as adverse side effects in patients with pigmentary retinal dystrophies.[14,15] Recently, a study investigated its efficacy on multifocal electroretinography (mfERG) and found a significant improvement in amplitude and latency/implicit time in mfERG.[16]
The purpose of our study was to further examine the safety and efficacy of long-term VPA treatment in patients with RP, using mfERG and VF analyses (VFAs) as objective examinations.
Methods
In this study, we have analyzed the data from 48 eyes of 24 patients (13 males, 11 females) with RP who were offered off-label use of VPA. The study was conducted in accordance with the ethical standards stated in the 1964 Declaration of Helsinki and approved by the local ethics committee. Informed, written consent was obtained from all participants.
A diagnosis of RP was made in patients presenting to our clinic on the basis of history of night blindness and clinical signs such as waxy pallor of the optic nerve, vascular attenuation, and/or the presence of intraretinal pigment. Patients with nonsyndromic RP, without any systemic association, cooperative for mfERG were included. Patients with atypical RP (e.g., sectoral, pericentral, or inverse), media opacities, cystoid macular edema (confirmed on ophthalmoscopy and optical coherence tomography), glaucoma, nystagmus, myopia >−6.00 diopter sphere, or any systemic disease that could affect vision or their capacity to perform the tests were excluded.
The dosage of VPA was 500 mg/day, which is much lower than the anticonvulsant dose. Before VPA treatment, the potential risks, benefits, and alternatives were discussed with all patients. Blood chemistry, including serum liver enzymes, electrolyte and blood cell panels, and reported side effects (tiredness, stomach irritation, weight gain, alopecia), was assessed at baseline and during treatment.
All patients underwent complete examinations including slit-lamp biomicroscopy, fundus examination, best-corrected VA (BCVA) measurement, VFA, and mfERG examination.
BCVA was recorded using a Snellen chart at a distance of 6.1 m. Values were converted to the logarithm of the minimum angle of resolution (logMAR) score for statistical analysis.
Regarding interpretation of VFA (Central 24-2 Full Threshold Test with white target by Humprey automated perimetry), mean deviation (MD) and pattern standard deviation (PSD) were evaluated. Based on the manufacturer's recommendations, test results were considered reliable if fixation loss and false negative and false positive rates were >30%.
mfERG was performed using Roland-RETI scan system (Roland Consult, Brandenburg, Germany) under the guidelines given by the International Society for Clinical Electrophysiology for Vision.[17] Stimulation source used was CRT monitor (17″ color monitor, luminance 80 cd/m2, high contrast; Roland Consult) with frame frequency of 75 Hz. Stimulation calibrations were done as provided by the RETI scan software (Roland Consult). The high-pass cutoff was 10 Hz and low was 100 Hz. The artifact level was 10%. The records obtained were analyzed in terms of the grouped data as group averages. The averages were taken in terms of the concentric rings. Duration of the test time was eight cycles. Fixation was meticulously monitored during the testing duration to prevent abnormal mfERG findings due to voluntary eccentric fixation. For data analysis, the mfERG responses were grouped into five concentric rings, from the center outward: ring 1 to the fovea; ring 2 to the parafovea; ring 3 to the perifovea; ring 4 to the near periphery; and ring 5 to the central part of the middle periphery.
Statistical analyses were calculated using SPSS software (version 21.0, SPSS, Inc., Chicago, IL, USA). The data were normally distributed, met by the Kolmogorov–Smirnov test (P > 0.05). The results are presented as the mean ± the standard deviation. A paired t-test was used to compare the measurements before and after VPA treatment. P < 0.05 was considered statistically significant.
Results
Mean age of the patients was 34.3 ± 10.3 years (range 18–56 years). The length of treatment was 9.4 ± 2.7 months (range 6–12 months). The mean BCVA before and after VPA therapy was 0.36 ± 0.38 and 0.36 ± 0.37 logMAR, respectively, and was not different statistically (P = 0.32). Overall, 40 eyes had no change in VA, six eyes had a decline in VA, and two eyes showed improvement (>1 line in decimal) in VA [Table 1].
Table 1.
Best-corrected visual acuity and visual field analyses results before and after valproic acid treatment
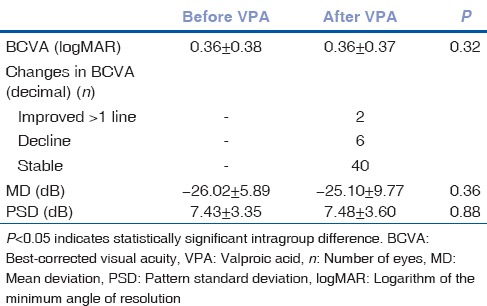
Fifteen of the patients (30 eyes) had two mfERG and VFA tracings allowing comparison between baseline and follow-up. Quantitative perimetric indices of MD (dB) values before and after VPA were − 26.02 ± 5.89 and − 25.10 ± 9.77, respectively, and the difference was not statistically significant (P = 0.36). The mean PSD (dB) before and after VPA was 7.43 ± 3.35 and 7.48 ± 3.60, respectively, and was not significantly different (P = 0.88) [Table 1].
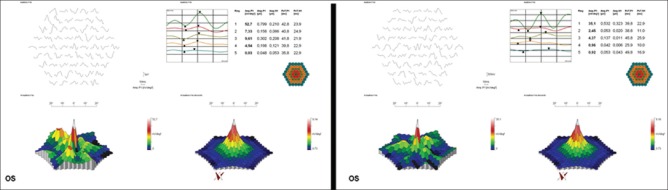
Table 2 shows the mfERG results for all rings. P1 amplitudes (in terms of nV/deg2 and mV) of ERG waves were significantly decreased in the rings 1, 3, and 4 after VPA therapy (P < 0.05). However, their P1 latencies were not significantly changed in all the rings after VPA therapy (P > 0.05). Regarding the N1 amplitudes, the only significant decrease was observed in area 1 (P = 0.03). Similarly, there was no significant change in N1 latencies in any of the rings (P > 0.05), except the significant increase in area 3 after VPA therapy (P = 0.04) [Fig. 1].
Table 2.
Comparison of multifocal electroretinography parameters before and after valproic acid treatment
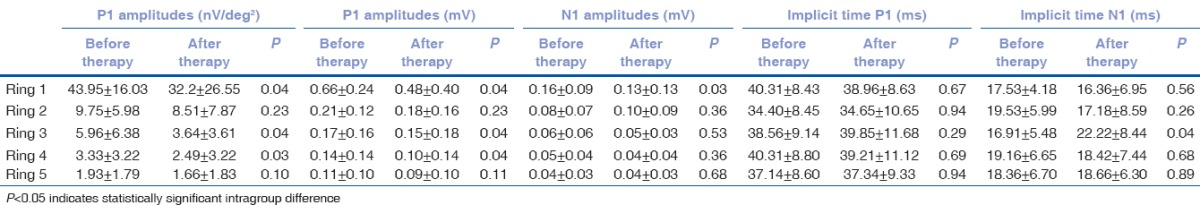
Figure 1.
Multifocal electroretinography of a patient before (left) and after (right) valproic acid treatment. No significant improvement is shown in any of the rings after valproic acid therapy
None of the patients had abnormal liver function or blood chemistries. The most common side effects were tiredness (12.5%), stomach irritation (8.3%), weight gain (4.1%), and alopecia (4.1%).
Discussion
RP is a blinding disease with no robust treatment options. VPA is widely used as an anticonvulsant and mood stabilizer, and its efficacy in these capacities is probably mediated through its ability to affect GABA levels through glutamic acid decarboxylase and GABA transaminase modulation.[18,19] A particularly exciting property of VPA has recently been documented, suggesting that it has the unique ability to reverse photoreceptor damage. VPA can induce cells to differentiate in culture.[6] Moreover, VPA has been shown to stimulate glial cells to differentiate into photoreceptor-like cells.[20]
A limited number of studies evaluated the efficacy and safety of VPA in RP. Clemson et al. examined 13 eyes before and after brief treatment (average 4 months) with VPA.[13] They found that nine eyes had improved VFA with treatment, two eyes had decreased VFA, and two eyes experienced no change, with an overall average increase of 11%. They also assigned a significant decrease in the logMAR scores in these 13 eyes, assuming no loss in acuity without treatment with mild- and well-tolerated side effects.
Following Clemson et al., Sisk reported VPA treatment-associated toxicity and intolerable side effects in three patients with nondominant RP.[13,14] Sisk suggested that although VPA has been described as having anti-inflammatory and neuroprotective properties, it may instead be toxic to eyes with RP that is not caused by mutations affecting rhodopsin. VPA blocks voltage-gated sodium channels and T-type calcium channels,[21] diminishes high-frequency repetitive firing of action potentials of central neurons in culture, and may have a role in diminishing hyperpolarization of photoreceptors, which fire continuously except when stimulated, by decreasing the standing potential. This theoretically may compromise photoreceptor function in eyes with recessive or sporadic RP to account for the observed visual decline and failure to recover after discontinuation of the drug.
In another study, Bhalla et al. performed a retrospective study on 31 patients with various pigmentary retinal dystrophies after an average of 9.8 months.[15] In contrast to the Clemson publication, VFA areas showed a declining trend in four out of five patients and average VA significantly worsened during treatment with VPA (P = 0.002). In addition, VPA was associated with adverse side effects in their patients. Recently, Iraha et al. found that the VFA showed improvements during the 6-month follow-up; however, these were reversed to the baseline values after interruption of the drug.[22]
In our study, we used mfERG as a more sensitive and objective examination than VA or VFs, for the evaluation of our functional results. mfERG selects the electrophysiological responses of multiple retinal locations of the macular and perimacular area, which are tested simultaneously, allowing functional mapping of the central retina. The only study evaluating the effect of VPA on mfERG was reported by Kumar et al.[16] At 1-year follow-up, 14 of 15 patients in VPA group showed a statistically significant improvement in amplitude and latency/implicit time in mfERG (P < 0.001).
However, our mfERG data show a statistically significant decrease in P1 amplitudes in the rings 1, 3, and 4, following the average 9 months of VPA treatment. Furthermore, no significant improvement was found for any of the mfERG parameters after VPA therapy. In addition, we have not found any significant benefit of VPA treatment on the mean BCVA and VFA indices. There may be some explanations for these results in addition to those suggested before.[14,21] It may be related with the mitochondrial toxicity of VPA. The mitochondrial adverse events associated with VPA use include inhibition and decreased activity of mitochondrial complexes I and IV, inhibition of oxygen consumption and adenosine triphosphate synthesis, sequestration of coenzyme A, impaired structural organization of the inner mitochondrial membrane, depleted hepatic cytochrome aa3, impaired oxidative phosphorylation, inhibition of mitochondrial β-oxidation, and vacuolar fragmentation.[11,12,22,23] However, these adverse effects were often described for higher dosages used for other indications such as anticonvulsant activity (25–40 mg/kg/day). Another explanation may be that RP patients are expected to show 6%–10% reduction in mfERG amplitudes annually.[24]
The multiple metabolic pathways involved in VPA biotransformation give rise to more than 50 known metabolites of the parent drug which may cause a spectrum of side effects and result central nervous system toxicity in higher doses.[11,12] We, however, observed no significant side effects in our patients undergoing therapy with VPA, except for tiredness (12.5%), stomach irritation (8.3%), weight gain (4.1%), and alopecia (4.1%).
There are some limitations in the current study. The patients were not genetically characterized, and genetic variation in RP might account for variability in the therapeutic response to VPA. In addition, we did not compare our data with those of a control group. However, the dose which we have given in this study is very low compared to dosage given in neurological conditions, so we may conclude that VPA may have some adverse effects in visual functions in RP patients. Finally, though this study is of a prospective nature, patients were not administered a drug for a fixed period due to their inconsonance. Therefore, the treatment period was not standard in all cases.
Conclusion
In the current study, we prospectively examined 48 eyes of RP patients using VPA for a long-term period. After an average of 9 months of VPA treatment, we have not found any significant benefit on the patients’ mean BCVA and VFA indices. Indeed, VPA treatment was associated with decline in some mfERG parameters. Therefore, physicians should avoid prescribing VPA for RP until the safety and efficacy of this treatment are appropriately evaluated in the further prospective studies using larger study populations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 2.Berson EL, Rosner B, Weigel-DiFranco C, Dryja TP, Sandberg MA. Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations. Invest Ophthalmol Vis Sci. 2002;43:3027–36. [PubMed] [Google Scholar]
- 3.Hoffman DR, Locke KG, Wheaton DH, Fish GE, Spencer R, Birch DG, et al. A randomized, placebo-controlled clinical trial of docosahexaenoic acid supplementation for X-linked retinitis pigmentosa. Am J Ophthalmol. 2004;137:704–18. doi: 10.1016/j.ajo.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 4.Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37(Suppl 2):5–16. [PubMed] [Google Scholar]
- 6.Göttlicher M, Minucci S, Zhu P, Krämer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–78. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Pang H, et al. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience. 2007;149:203–12. doi: 10.1016/j.neuroscience.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dragunow M, Greenwood JM, Cameron RE, Narayan PJ, O’Carroll SJ, Pearson AG, et al. Valproic acid induces caspase 3-mediated apoptosis in microglial cells. Neuroscience. 2006;140:1149–56. doi: 10.1016/j.neuroscience.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 9.Suuronen T, Nuutinen T, Ryhänen T, Kaarniranta K, Salminen A. Epigenetic regulation of clusterin/apolipoprotein J expression in retinal pigment epithelial cells. Biochem Biophys Res Commun. 2007;357:397–401. doi: 10.1016/j.bbrc.2007.03.135. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry. 2009;14:51–9. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- 11.Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46:1323–38. doi: 10.1016/j.clinbiochem.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Silva MF, Aires CC, Luis PB, Ruiter JP, IJIst L, Duran M, et al. Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: A review. J Inherit Metab Dis. 2008;31:205–16. doi: 10.1007/s10545-008-0841-x. [DOI] [PubMed] [Google Scholar]
- 13.Clemson CM, Tzekov R, Krebs M, Checchi JM, Bigelow C, Kaushal S, et al. Therapeutic potential of valproic acid for retinitis pigmentosa. Br J Ophthalmol. 2011;95:89–93. doi: 10.1136/bjo.2009.175356. [DOI] [PubMed] [Google Scholar]
- 14.Sisk RA. Valproic acid treatment may be harmful in non-dominant forms of retinitis pigmentosa. Br J Ophthalmol. 2012;96:1154–5. doi: 10.1136/bjophthalmol-2012-301950. [DOI] [PubMed] [Google Scholar]
- 15.Bhalla S, Joshi D, Bhullar S, Kasuga D, Park Y, Kay CN, et al. Long-term follow-up for efficacy and safety of treatment of retinitis pigmentosa with valproic acid. Br J Ophthalmol. 2013;97:895–9. doi: 10.1136/bjophthalmol-2013-303084. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Midha N, Gogia V, Gupta S, Sehra S, Chohan A, et al. Efficacy of oral valproic acid in patients with retinitis pigmentosa. J Ocul Pharmacol Ther. 2014;30:580–6. doi: 10.1089/jop.2013.0166. [DOI] [PubMed] [Google Scholar]
- 17.Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, et al. ISCEV guidelines for clinical multifocal electroretinography (2007 edition) Doc Ophthalmol. 2008;116:1–11. doi: 10.1007/s10633-007-9089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman A, Keane PE, Meldrum BS, Simiand J, Vernieres JC. Mechanism of anticonvulsant action of valproate. Prog Neurobiol. 1982;19:315–59. doi: 10.1016/0301-0082(82)90010-7. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald RL, Bergey GK. Valproic acid augments GABA-mediated postsynaptic inhibition in cultured mammalian neurons. Brain Res. 1979;170:558–62. doi: 10.1016/0006-8993(79)90975-2. [DOI] [PubMed] [Google Scholar]
- 20.Kubota A, Nishida K, Nakashima K, Tano Y. Conversion of mammalian müller glial cells into a neuronal lineage by in vitro aggregate-culture. Biochem Biophys Res Commun. 2006;351:514–20. doi: 10.1016/j.bbrc.2006.10.072. [DOI] [PubMed] [Google Scholar]
- 21.Löscher W. Basic pharmacology of valproate: A review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–94. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 22.Iraha S, Hirami Y, Ota S, Sunagawa GA, Mandai M, Tanihara H, et al. Efficacy of valproic acid for retinitis pigmentosa patients: A pilot study. Clin Ophthalmol. 2016;10:1375–84. doi: 10.2147/OPTH.S109995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finsterer J, Zarrouk Mahjoub S. Mitochondrial toxicity of antiepileptic drugs and their tolerability in mitochondrial disorders. Expert Opin Drug Metab Toxicol. 2012;8:71–9. doi: 10.1517/17425255.2012.644535. [DOI] [PubMed] [Google Scholar]
- 24.Nagy D, Schönfisch B, Zrenner E, Jägle H. Long-term follow-up of retinitis pigmentosa patients with multifocal electroretinography. Invest Ophthalmol Vis Sci. 2008;49:4664–71. doi: 10.1167/iovs.07-1360. [DOI] [PubMed] [Google Scholar]