Abstract
Purpose
Cromakalim prodrug 1 (CKLP1) is a water-soluble ATP-sensitive potassium channel opener that has shown ocular hypotensive properties in ex vivo and in vivo experimental models. To determine its mechanism of action, we assessed the effect of CKLP1 on aqueous humor dynamics and in combination therapy with existing ocular hypotensive agents.
Methods
Outflow facility was assessed in C57BL/6 mice by ex vivo eye perfusions and by in vivo constant flow infusion following CKLP1 treatment. Human anterior segments with no trabecular meshwork were evaluated for effect on pressure following CKLP1 treatment. CKLP1 alone and in combination with latanoprost, timolol, and Rho kinase inhibitor Y27632 were evaluated for effect on intraocular pressure in C57BL/6 mice and Dutch-belted pigmented rabbits.
Results
CKLP1 lowered episcleral venous pressure (control: 8.9 ± 0.1 mm Hg versus treated: 6.2 ± 0.1 mm Hg, P < 0.0001) but had no detectable effect on outflow facility, aqueous humor flow rate, or uveoscleral outflow. Treatment with CKLP1 in human anterior segments without the trabecular meshwork resulted in a 50% ± 9% decrease in pressure, suggesting an effect on the distal portion of the conventional outflow pathway. CKLP1 worked additively with latanoprost, timolol, and Y27632 to lower IOP, presumably owing to combined effects on different aspects of aqueous humor dynamics.
Conclusions
CKLP1 lowered intraocular pressure by reducing episcleral venous pressure and lowering distal outflow resistance in the conventional outflow pathway. Owing to this unique mechanism of action, CKLP1 works in an additive manner to lower intraocular pressure with latanoprost, timolol, and Rho kinase inhibitor Y27632.
Keywords: ATP-sensitive potassium channel, intraocular pressure, glaucoma medications, distal outflow, ocular hypertension
Glaucoma is a progressive neurodegenerative disorder of the eye, characterized by loss of retinal ganglion cells and an increased cup to disc ratio at the optic nerve head.1 Intraocular pressure (IOP) is often increased owing to pathologic changes in the trabecular meshwork (the major pathway for egress of aqueous humor from the anterior chamber), which causes increased resistance to aqueous humor outflow. Owing to an increasing aging population, the incidence of glaucoma is expected to rise by 40% by 2040.2 All current clinical strategies for treating glaucoma are aimed at lowering IOP, a method that slows disease progression.3–9 Several major studies10–13 have indicated that lowering IOP can also be effective in slowing down visual field loss in glaucoma patients with IOP in the normal physiologic range (normal tension glaucoma).
Pharmacologic agents used to treat glaucoma lower IOP by either reducing the rate of production of aqueous humor from the ciliary body (e.g., α-2 adrenergic agonists like brimonidine, apraclonidine; β-blockers like timolol), or by increasing aqueous flow through a secondary pathway called the uveoscleral outflow pathway (e.g., F-class prostaglandin analogs like latanoprost, bimatoprost, and travoprost).14–16 Some drugs (e.g., pilocarpine, rho kinase inhibitors) also decrease the resistance to aqueous flow through the trabecular meshwork (i.e., increase outflow facility). Unfortunately, all existing glaucoma therapeutics have side effects ranging from mild local discomfort to severe systemic episodes.16–19 Use of multiple drugs to lower IOP is often necessary, which further exacerbates the side effect profile. Additionally, none of the existing glaucoma drugs other than the rho kinase inhibitors (currently approved for clinical use only in Japan) have a primary effect on the cells and tissues of the conventional outflow pathway (e.g., trabecular meshwork, Schlemm's canal).14,20 New glaucoma drugs (e.g., adenosine receptor agonists and modified prostaglandin analogs) purportedly targeting the conventional outflow pathway are being tested in various preclinical and clinical trials in the United States.21–36 However, even these newer drugs show a host of adverse side effects, ranging from conjunctival hyperemia and pain at the site of instillation, to events like headache and oropharyngeal pain.21,26,28,37 Because of this, and because over time patients usually become refractory to topical glaucoma medications, continued search for the development of therapeutics with a unique mechanism of action and reduced or nonexistent side effect profile is a significant need for clinicians treating glaucoma.14,38,39
Our laboratory has established a novel ocular hypotensive property of several ATP-sensitive potassium (KATP) channel openers in ex vivo and in vivo model systems.19,40–42 Commercially available openers of KATP channels have minimal aqueous solubility and generally dissolve in organic solvents (e.g., dimethyl sulfoxide), making them unsuitable for application to human eyes. We have recently developed a novel prodrug with aqueous solubility, called cromakalim prodrug 1 (CKLP1), based on the structure of the KATP channel opener cromakalim.43 CKLP1 retains the IOP-lowering properties of cromakalim and is well tolerated by experimental animals (e.g., mice and rabbits) with no observable local or systemic side effects.43 The mechanism by which CKLP1 lowers IOP is unknown. We hypothesized that CKLP1 affects one or more parameters of aqueous humor dynamics, resulting in reduced IOP. In the current study, we evaluated the effect of CKLP1 on aqueous humor dynamics in mice and validated the findings in ex vivo human anterior segment perfusion cultures. The feasibility of using CKLP1 to lower IOP in combination with known ocular hypotensive agents in normotensive mouse and rabbit models was also assessed.
Methods
Reagents
CKLP1 was synthesized in-house, according to previously published methods (originally described as [3S,4R]-2) and dissolved in phosphate-buffered saline (PBS).43 Latanoprost free acid (LFA) was purchased from Cayman Chemicals (Ann Arbor, MI, USA) in methyl acetate. Methyl acetate was removed by evaporation under a gentle stream of liquid nitrogen to obtain pure LFA. This was subsequently dissolved in dimethyl sulfoxide to obtain a stock concentration of 10−1 M LFA and further diluted 1000-fold in PBS to obtain a working concentration of 10−4 M. Timolol was obtained as timolol maleate from Sigma-Aldrich Corp. (St. Louis, MO, USA) and Rho kinase inhibitor Y27632 was purchased from Enzo Life Sciences (Farmingdale, NY, USA). Both Timolol (0.5%) and Y27632 (10 mM) were dissolved in PBS to their final working concentrations.
Animal Care
All experimental protocols with animals were preapproved by the animal ethics committees of the respective institutions where the studies were performed (Mayo Clinic, Rochester, MN, USA; Imperial College, London, UK; North Texas Eye Research Institute, Fort Worth, TX, USA) and adhered to the tenets of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Studies at Imperial College London were conducted under the authority of a UK Home Office project license. Mice were housed with five or fewer mice per cage, fed with standard rodent chow, given water ad libitum, and maintained in pathogen-free facilities with 12-hour light and dark cycles. Rabbits were housed two to a cage in a climate-controlled room with 12-hour light and dark cycles and fed standard rabbit food pellets and given water ad libitum. Following delivery, mice and rabbits were acclimated to their new habitats for at least 5 days before initiating an experiment. Since animals were treated with topical eye drops, ocular (swelling, redness, discharge) and systemic (general behavior, food and water intake) health were routinely monitored by laboratory personnel and veterinary technicians (5 days per week).
IOP Measurement in Animal Models
IOP was measured in nonanesthetized mice and rabbits by using schedules and methods previously described.40,41 Briefly, animals were habituated to handling and IOP measurements for at least 2 days following acclimatization to the institutional animal facility. All IOP measurements were performed with a handheld rebound tonometer (Icare tonolab for mouse, Icare tonovet for rabbits; Colonial Medical Supply, Franconia, NH, USA), which had been precalibrated by the manufacturer for measurement of IOP in the respective animal species. For each IOP measurement, the tonometer probe strikes the center of the cornea six times and calculates IOP by using an algorithm based on probe incident velocity and deceleration. A total of three independent readings at any given time point were averaged to obtain the final IOP reading. For pretreatment, treatment, and posttreatment periods, IOP was measured daily three times corresponding to 1, 4, and 23 hours following treatment (approximately 10:00 AM, 2:00 PM, and the following morning at 9:00 AM). Daily IOP was expressed as the mean ± standard deviation of all three time points for a given day.
For the ex vivo perfusion studies in which CKLP1 was delivered topically before death (see below), IOP was measured between 10:00 AM and 12:00 PM under isoflurane anesthesia, before applying each topical dose of CKLP1. IOP was measured with a rebound tonometer mounted on a retort stand with the probe aligned along the visual axis. IOP was measured three times, each measurement consisting of six rebounds, and the average of the three measurements was used as the daily IOP.
Ex Vivo Measurement of Outflow Facility
Two perfusion experiments were performed with 13-week-old male C57BL/6 mice (Charles River UK Ltd., Margate, UK), where either CKLP1 was perfused directly into the conventional outflow pathway of enucleated mouse eyes or CKLP1 was applied topically in vivo followed by ex vivo perfusion. For the former set of experiments, six mice were humanely culled by cervical dislocation. Eyes were enucleated within 10 to 15 minutes of death, and outflow facility was measured simultaneously in paired eyes by using a dual channel iPerfusion system, as previously described.44,45 Briefly, eyes were glued to a support platform and fully immersed in PBS regulated at 35°C. Anterior chambers were cannulated with a 33-gauge needle connected to a micromanipulator, and equilibrated at 9 mm Hg for 30 minutes. The perfusate comprised sterile filtered Dulbecco's PBS including divalent cations and 5.5 mM glucose (collectively referred to as DBG). Experimental eyes were perfused with either 5 mM (n = 2) or 10 mM (n = 3) CKLP1 in DBG, while contralateral eyes were perfused with DBG alone. Pressure was raised by using a motorized reservoir over seven increasing steps from 6.5 to 17 mm Hg. For each pressure step, flow was considered to have reached steady state when the rate of change of the flow rate to pressure (averaged over a 5-minute window) was below 0.1 nL/min/mm Hg/min for 1 minute. The last 4 minutes of data were then used to calculate the mean steady-state flow rate and pressure. Pressure steps that did not achieve steady state were excluded from further analysis, and any pair where at least one eye did not reach steady state for four or more steps was excluded. In this study, five of the six pairs met the stability criteria. The “n” values refer to the mice that met the criteria (n = 2 for 5 mM and n = 3 for 10 mM). Steady-state flow rate 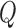 and pressure
and pressure 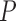 for each eye was then fit by the relationship
for each eye was then fit by the relationship
 |
where 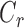 is the outflow facility at a reference pressure
is the outflow facility at a reference pressure 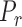 defined to be 8 mm Hg, and
defined to be 8 mm Hg, and 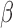 characterizes the nonlinearity of the
characterizes the nonlinearity of the 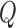 –
–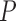 relationship.
relationship. 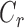 was compared between paired eyes by using a weighted t-test as previously described.44,45
was compared between paired eyes by using a weighted t-test as previously described.44,45
For the second set of experiments, eight mice were treated with CKLP1 (2.5 mM in PBS) by unilateral eye drops (10 μL) given daily under general anesthesia for 4 consecutive days. Contralateral eyes were treated with vehicle (PBS). IOP was measured with a mounted rebound tonometer as described above. Following the fourth dose (delivered 72 hours after the first), mice were killed by cervical dislocation within 1 hour, and eyes were enucleated within 10 minutes of death. Outflow facility was measured in paired, enucleated eyes by using the iPerfusion system as described above.44,45 CKLP1 was not included in the perfusion fluid. In this study, six of the eight pairs met the stability criteria and were included in the final analysis.
In Vivo Evaluation of Aqueous Humor Dynamics by Constant Flow Infusion
Wild-type C57BL/6J mice (n = 12) were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Following 3 days of baseline IOP measurements, a 5-μL bolus of CKLP1 (5 mM) was applied topically to one eye of each animal, while the contralateral eye received a 5-μL bolus of vehicle (PBS) once daily for 5 consecutive days. IOP was measured at 1, 4, and 23 hours daily after each treatment. Following day 5 of treatment, animals were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), and aqueous humor dynamics were assessed by constant flow infusion as previously described.46,47 Briefly, a 30-gauge needle connected to a calibrated sphygmomanometer (Diagnostix 700; American Diagnostic Corporation, Hauppauge, NY, USA) attached to a BLPR-2 pressure transducer (World Precision Instruments, Sarasota, FL, USA) was inserted into the anterior chambers of mouse eyes. The opposing end of the transducer was connected to a three-way valve attached to a 50-μL glass microsyringe (Hamilton Company, Reno, NV, USA) filled with PBS and loaded onto an SP101i microdialysis infusion pump (World Precision Instruments), and a variable-height open-ended manometer. The BLPR-2 transducer was connected electrically to a TBM4M Bridge Amplifier and a Lab-Trax analog to digital converter (World Precision Instruments). All data were fed into a computer, recorded, and analyzed with LabScribe2 software (World Precision Instruments). Outflow facility and episcleral venous pressure were measured, and uveoscleral outflow and aqueous humor formation rate were estimated as previously described.46,47
Human Anterior Segment Perfusion Culture
Use of human tissue for this study was approved by the Mayo Clinic Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. Human donor eyes without documented eye history (n = 4; 1 male and 3 females; age, 54.3 ± 17.7 years) were obtained from the Minnesota Lions Eye Bank (St. Paul, MN, USA), bisected at the equator; stripped of iris, ciliary body, and trabecular meshwork; and placed in modified petri dishes attached to a custom-designed pressure recording system within 14.4 ± 9.0 hours from death, as previously described.40,42,48 Eyes were maintained at 37°C and allowed to reach stable baseline pressure under perfusion with Dulbecco's modified Eagle's medium (DMEM) containing 1% antibiotic/antimycotic solution (Sigma-Aldrich), at the normal human aqueous humor flow rate of 2.5 μL/min. As soon as eyes reached stable pressure (2–4 days), DMEM containing cromakalim (2 μM) was perfused in one eye while the contralateral eye received vehicle. Hourly pressure readings were obtained by averaging sixty 1-minute measurements. Outflow facility was calculated by dividing the flow rate by pressure at 24 hours following treatment.
Histology of Human Anterior Segments
Viability of the human anterior segment cultures, as well as the extent of trabecular meshwork tissue removal, was evaluated by transmission electron microscopy according to previously described methods.40 Briefly, tissue wedges containing the trabecular meshwork and Schlemm's canal were isolated from each eye, fixed in 4% paraformaldehyde dissolved in 0.1 M phosphate buffer (pH 7.2), postfixed in 2% osmium tetroxide (Electron Microscopy Sciences, Hatfield, PA, USA) dissolved in 0.1 M phosphate buffer, and dehydrated in ascending alcohol concentrations. Tissues were cleared by using acetone (Fisher Chemical, Fair Lawn, NJ, USA), embedded in epoxy resin, and sectioned by using an ultramicrotome (Leica Microsystems, Buffalo Grove, IL, USA). Sections (100 μm) were subsequently stained with toluidine blue for light microscopy or mounted on copper grids and stained with 2% uranyl acetate (Electron Microscopy Sciences) and lead citrate (Mager Scientific, Dexter, MI, USA) for evaluation under a JEOL 1400 transmission electron microscope (JEOL USA, Peabody, MA, USA).
Combination Drug Treatment in Kir6.2(−/−) Mice
Wild-type C57BL/6 mice (6–8 months old, n = 10) obtained from Charles River, USA (Wilmington, MA, USA) and Kir6.2(−/−) mice (6–8 months old; generous gift from Andre Terzic, Mayo Clinic, Rochester, MN, USA) were measured for baseline IOP as described above. One eye of each mouse was treated with CKLP1 (5 mM, 5-μL bolus) once daily for 5 days, while the contralateral eye received vehicle. After 5 days of treatment, the CKLP1-treated eye was treated with CKLP1+LFA (10−4 M) once daily for 5 additional days, while the contralateral eye received vehicle. IOPs were measured three times daily throughout the treatment period (1, 4, and 23 hours following treatment). At the end of 5 days, combination treatment was stopped but IOP measurements were taken for 3 additional days. The final working concentration of LFA (10−4 M) is similar to commercially available formulations of latanoprost and has been shown to be effective in normotensive mice.40
Combination Drug Treatment With CKLP1 in Rabbits
Dutch-belted pigmented rabbits (6–7 months old) were purchased from Covance (Covance Research Products, Inc., Denver, PA, USA). Before initiation of an experiment, IOP was measured in rabbits at least twice daily for 2 to 3 days to habituate them to handling and IOP measurement techniques.
Study 1: Dose Response
Optimal CKLP1 dose for IOP reduction was obtained by treating one eye of each rabbit (n = 10) with increasing doses of CKLP1 (1 mM, 2.5 mM, 5 mM, 10 mM, 20 mM), while the contralateral eye received vehicle (PBS). Treatment was initiated following 3 days of baseline IOP measurements. Each concentration of CKLP1 was topically added to the eye in a 50 μL-bolus at the same time each day for 5 consecutive days before moving on to the next higher dose.
Study 2: Combination Drug Treatment
Rabbits were treated with CKLP1 (10 mM) and LFA (10−4 M, aqueous humor half-life 2–3 hours)49,50 either alone or in combination. Following 3 days of baseline IOP measurements, each rabbit was treated in one eye with CKLP1 for 5 days, while the fellow eye received appropriate vehicle. After 5 days of treatment, the eye treated with CKLP1 was treated with CKLP1+LFA for 5 days, LFA alone for 5 days, and LFA+CKLP1 for 5 days. IOP measurements were performed daily. After cessation of treatments, IOP was measured in each eye for 3 additional days to determine if IOP returned to baseline. Combination treatment with CKLP1+timolol (0.5%, aqueous humor half-life 4 hours)50 and CKLP1+Rho kinase inhibitor Y27632 (10 mM, plasma half-life 10–16 hours)51,52 was performed with the same experimental design. Use of timolol at 0.5% and LFA at 10−4 M was based on the amount of the drugs available in commercial formulations. The administered dose of Y27632 was based on a previous report in which 10 mM has been shown to produce significant reduction in IOP in rabbits.36
Statistical Analysis
All values are represented as mean ± standard deviation except for iPerfusion studies. Data for multiple CKLP1 doses and treatment groups in rabbits and mice were analyzed by ANOVA followed by Tukey's HSD (honest significant difference) test to identify means of groups that were significantly different from each other. Parameters related to aqueous humor dynamics obtained by constant infusion method in vivo in mice and ex vivo in human anterior segments were analyzed by two-tailed paired Student's t-test. Statistical analyses were performed with the JMP 10 software (SAS, Cary, NC, USA). For ex vivo mouse eye perfusion studies with iPerfusion, we report the average relative change in 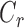 between contralateral treated and control eyes along with the 95% confidence interval (95% CI) on this relative change. Log-transformed data were used for statistical analysis, and significance was calculated by weighted t-test, as described previously,44,45 using Matlab (Mathworks, Natick, MA, USA).
between contralateral treated and control eyes along with the 95% confidence interval (95% CI) on this relative change. Log-transformed data were used for statistical analysis, and significance was calculated by weighted t-test, as described previously,44,45 using Matlab (Mathworks, Natick, MA, USA).
Results
Effect of CKLP1 on Aqueous Humor Dynamics
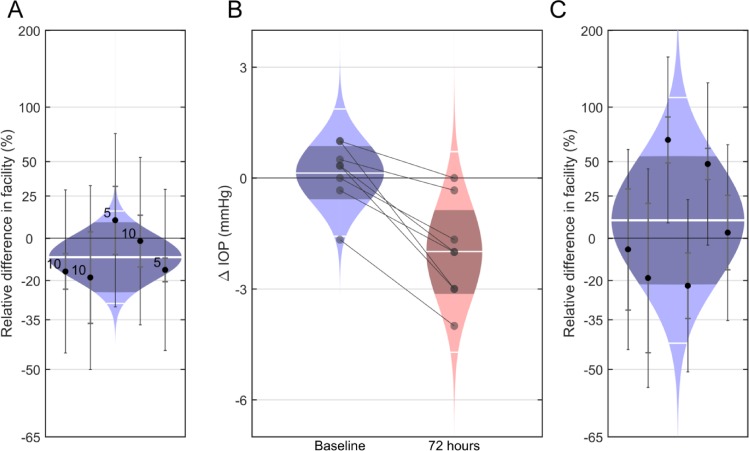
We first evaluated the effect of CKLP1 on outflow facility, with the hypothesis that CKLP1 would increase outflow facility owing to its ocular hypotensive properties. We measured outflow facility by directly perfusing CKLP1 into enucleated mouse eyes. In response to either 5 or 10 mM CKLP1, 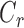 was unchanged with an average difference of −10% [−25% to +8%] (mean [95% CI]; P = 0.2, n = 5; Fig. 1A). Because CKLP1 must be activated by phosphatases present within the cornea, we reasoned that CKLP1 may not be sufficiently activated when perfused directly into enucleated eyes. Therefore, a second set of experiments measured outflow facility ex vivo following topical application of CKLP1 over several days. For these studies, one eye of each mouse was treated daily for 4 days with CKLP1, while the contralateral eye received vehicle control. IOP in treated eyes was reduced, with an average change of −2.0 [−3.1 to −0.9] mm Hg (mean [95% CI]; P = 0.004; n = 8 mice) relative to contralateral control eye at 72 hours (Fig. 1B). However, outflow facility of the treated eyes was not significantly different from the controls with a relative difference of 10% [−21% to 54%] (P = 0.5, N = 6; Fig. 1C). These results were further verified by direct perfusion with cromakalim (active parent compound of CKLP1, 40 μM) or with the dephosphorylated form of CKLP1 (20–80 μM) that yields the active compound cromakalim. Use of both cromakalim and the dephosphorylated form of CKLP1 did not show any significant changes in outflow facility of mouse eyes (Supplementary Fig. S1). Phosphatase digestion of CKLP1 and conversion to its parent compound was verified by mass spectrometry analysis (Supplementary Fig. S2; Methods). Together, these results do not lend support to our initial hypothesis that CKLP1 increases outflow facility.
was unchanged with an average difference of −10% [−25% to +8%] (mean [95% CI]; P = 0.2, n = 5; Fig. 1A). Because CKLP1 must be activated by phosphatases present within the cornea, we reasoned that CKLP1 may not be sufficiently activated when perfused directly into enucleated eyes. Therefore, a second set of experiments measured outflow facility ex vivo following topical application of CKLP1 over several days. For these studies, one eye of each mouse was treated daily for 4 days with CKLP1, while the contralateral eye received vehicle control. IOP in treated eyes was reduced, with an average change of −2.0 [−3.1 to −0.9] mm Hg (mean [95% CI]; P = 0.004; n = 8 mice) relative to contralateral control eye at 72 hours (Fig. 1B). However, outflow facility of the treated eyes was not significantly different from the controls with a relative difference of 10% [−21% to 54%] (P = 0.5, N = 6; Fig. 1C). These results were further verified by direct perfusion with cromakalim (active parent compound of CKLP1, 40 μM) or with the dephosphorylated form of CKLP1 (20–80 μM) that yields the active compound cromakalim. Use of both cromakalim and the dephosphorylated form of CKLP1 did not show any significant changes in outflow facility of mouse eyes (Supplementary Fig. S1). Phosphatase digestion of CKLP1 and conversion to its parent compound was verified by mass spectrometry analysis (Supplementary Fig. S2; Methods). Together, these results do not lend support to our initial hypothesis that CKLP1 increases outflow facility.
Figure 1.
Effect of CKLP1 on outflow facility in ex vivo mouse eye perfusion. (A) CKLP1 did not significantly affect outflow facility when perfused into enucleated eyes from C57BL/6 mice, with a relative change of −10% [−25% to 8%] (mean [95% CI]; P = 0.2, N = 5) between CKLP1 and vehicle-treated contralateral eyes. The labels “5” and “10” indicate the concentration in millimolar of CKLP1 in the perfusion fluid. (B) Daily topical application of 2.5 mM CKLP1 reduced IOP, with a change of −2.0 [−3.1 to −0.9] mm Hg (mean [95% CI]; P = 0.004; n = 8 mice) after 72 hours relative to the contralateral vehicle-treated eye. Each data point represents the difference in IOP between the treated and contralateral untreated eye for an individual mouse. (C) Outflow facility measured ex vivo shortly after the 72-hour time point from (B) was not significantly affected by CKLP1, with a difference of 10% [−21% to 54%] (P = 0.5, n = 6) relative to the vehicle-treated eye. In (A) and (C), each data point represents the relative difference in Cr for an individual mouse between CKLP1 and vehicle-treated contralateral eyes. The inner error bars on each data point represent the 95% CIs from the fitting to the equation, while the outer error bars represent the additional variability between contralateral eyes, estimated from paired untreated eyes in a previous study.44 Colored regions represent the best estimates of the sample distributions (log-normal in [A] and [C], normal in [B]), with the mean or geometric mean and two-sigma levels of the distributions shown by the central and peripheral horizontal white lines, respectively. Dark central bands represent the 95% CI on the means.
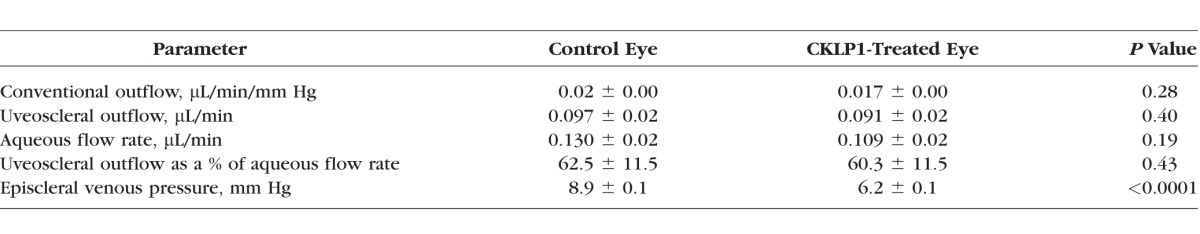
Since CKLP1 has shown robust IOP reduction in previous studies, we reasoned that the drug must be affecting other parameters of aqueous outflow dynamics. To test this, C57BL/6 mouse (n = 12) eyes were treated daily with vehicle and CKLP1 for 3 days. CKLP1 treatment resulted in a −3.3 ± 1.9 mm Hg change in IOP corresponding to a 24.6% ± 14.1% IOP drop as compared to paired control eyes (P < 0.0001). Using a constant perfusion method previously described by Millar et al.,46,47 we confirmed that CKLP1 had no effect on outflow facility (Table 1). Additionally, this model system also showed that CKLP1 did not alter aqueous humor flow rate or uveoscleral outflow. However, episcleral venous pressure was found to be significantly lowered in CKLP1 treated mouse eyes (control: 8.9 ± 0.1 mm Hg versus treated: 6.2 ± 0.1 mm Hg, P < 0.0001). This indicated that CKLP1 may lower IOP through a direct reduction of episcleral venous pressure.
Table 1.
Effect of CKLP1 on Various Parameters of Aqueous Humor Dynamics in Mouse Eyes
Effect of CKLP1 on Distal Outflow
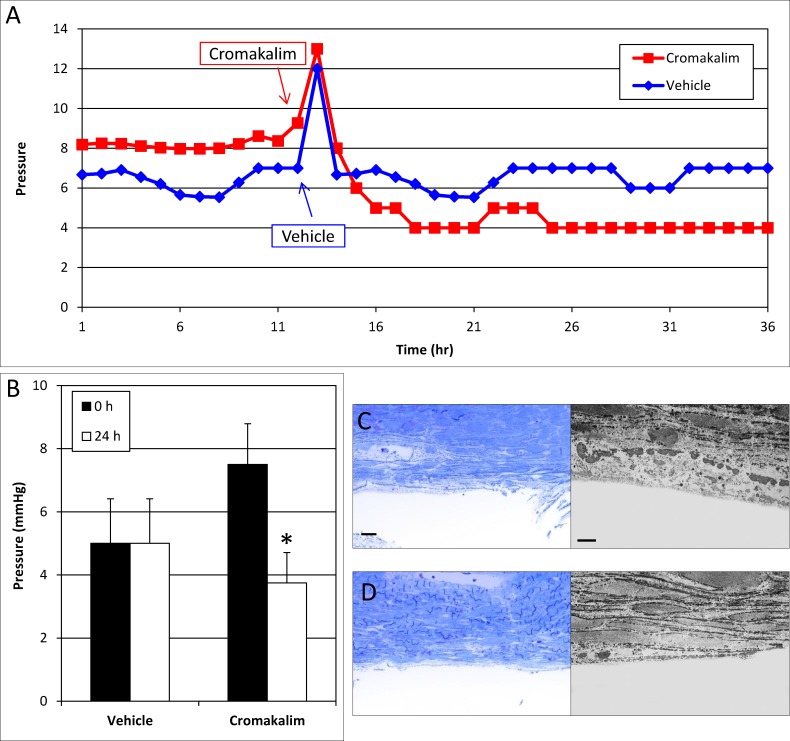
The effect on episcleral venous pressure was a surprising finding, especially since previous studies have found that cromakalim, the parent compound of CKLP1, lowers pressure in ex vivo human anterior segment culture. This model contains only the conventional outflow pathway and is generally used to study effects on the trabecular meshwork and Schlemm's canal.40 We hypothesized that the effect of cromakalim in ex vivo cultures may have occurred owing to a direct effect on the distal outflow portion of the conventional outflow pathway (e.g., collector channels, intrascleral venous plexus). To test this, we removed the trabecular meshwork in four pairs of eyes, allowed pressure to stabilize in culture, and treated one eye of each pair with cromakalim, the active component of CKLP1, and the fellow eye with vehicle. We did not use CKLP1 in these studies, since the drug is being perfused and may not be cleaved into its active form. When cromakalim was added to the perfused media, pressure dropped significantly in the treated eye (7.5 ± 1.3 mm Hg at 0 hour versus 3.8 ± 1.0 mm Hg at 24 hours, n = 4, P = 0.004), while no change was observed in the contralateral vehicle-treated eye (5.0 ± 1.4 mm Hg at 0 hour versus 5.0 ± 1.4 mm Hg at 24 hours) (Figs. 2A, 2B). This suggests that cromakalim lowers pressure in the absence of the trabecular meshwork by acting through components of the distal outflow pathway.
Figure 2.
Effect of cromakalim on intraocular pressure in human anterior segment perfusion culture following removal of the trabecular meshwork and parts of Schlemm's canal. (A) Representative graph of cromakalim treatment showing IOP reduction in human anterior segments with trabecular meshwork and parts of Schlemm's canal removed. (B) Treatment with cromakalim significantly lowered pressure from 7.5 ± 1.3 to 3.8 ± 1.0 mm Hg within 24 hours (P = 0.004), while pressure in the vehicle-treated contralateral eyes remained unchanged at 5.4 ± 1.4 mm Hg (n = 4). (C) Toluidine blue–stained thin sections (100 μm) and transmission electron micrographs of vehicle and (D) cromakalim-treated eyes show complete removal of the trabecular meshwork and the inner wall of Schlemm's canal. Only parts of the outer wall of Schlemm's canal can be seen. The remaining cells and tissues of the distal outflow pathway appear healthy and viable in both control and treated eyes. Scale bar: 20 μm for toluidine blue sections; 5 μm for transmission electron micrographs.
To confirm that human eye cultures were viable, all eyes were evaluated histologically. Toluidine blue sections and transmission electron micrographs showed complete removal of the trabecular meshwork along with the inner wall and parts of the outer wall of Schlemm's canal (Figs. 2C, 2D). Regions distal to the outer wall of Schlemm's canal appeared normal and healthy, indicating viable tissues in the cultured eyes.
Effect of Combination Treatment With CKLP1 and LFA in Mice
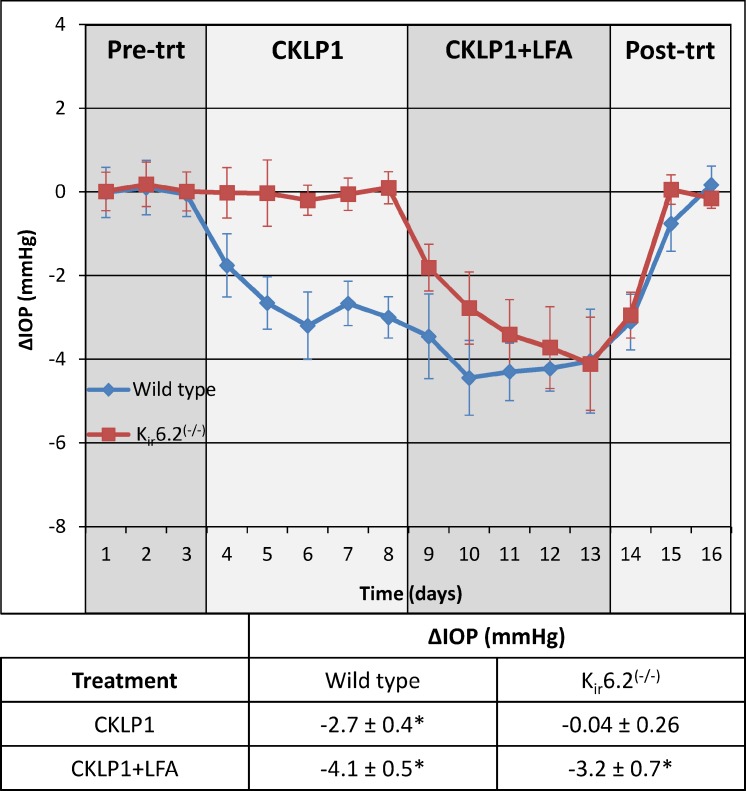
Owing to the unique effect of CKLP1 on episcleral venous pressure and distal outflow resistance, we reasoned that if used in conjunction with the glaucoma drug LFA, it may provide an additive effect on IOP reduction. To test this, we evaluated the effect of CKLP1 and CKLP1+LFA treatment in normotensive wild-type and Kir6.2(−/−) mice. Mice were first treated with topical eye drops of CKLP1, once daily for 5 consecutive days, followed by treatment with CKLP1+LFA for another 5 days. Treatment with CKLP1 reduced IOP by 16.1% ± 2.6% (−2.7 ± 0.4 mm Hg, P < 0.001) as compared to fellow control eyes (Fig. 3). However, combination treatment with LFA caused an additional 57.5% ± 20.2% drop in pressure (n = 10, P < 0.0001), corresponding to an overall IOP reduction of 25.1% ± 3.0% in the CKLP1+LFA-treated eye (−4.1 ± 0.5 mm Hg, P < 0.0001) compared to the control eye (Fig. 3). These data confirm an additive effect of CKLP1+LFA treatment in wild-type mice when compared to CKLP1 alone.
Figure 3.
Effect of CKLP1 on wild-type and Kir6.2(−/−) mice. CKLP1 caused significant reduction of IOP in wild-type (−2.7 ± 0.4 mm Hg, P < 0.001, blue line) but not Kir6.2(−/−) animals (−0.3 ± 1.6 mm Hg, P = 0.61, red line). Combination treatment with CKLP1+LFA showed an additive effect on IOP reduction in wild-type mice, and a 3.2 ± 0.7 mm Hg drop in IOP in the Kir6.2(−/−) mice. Since no IOP change was observed in Kir6.2(−/−) mice when treated with CKLP1 alone, drop in IOP observed after combination treatment with CKLP1+LFA was due to LFA. All values are mean ± standard deviation.
To address specificity, we treated Kir6.2(−/−) mice with CKLP1 alone and with the combination of CKLP1+LFA. Kir6.2 is an important KATP channel subunit, necessary for IOP reduction by cromakalim.19,40,41 CKLP1 treatment in Kir6.2(−/−) mice had no effect on IOP when compared to vehicle-treated control eyes (−0.04 ± 0.3 mm Hg, P = 0.61). However, combination treatment of CKLP1+LFA reduced IOP by 19.1% ± 4.1% (−3.2 ± 0.7 mm Hg, n = 10, P < 0.0001) as compared to control eyes (Fig. 3). These results suggest that similar to its parent compound cromakalim, CKLP1 has specificity for KATP channels containing Kir6.2 subunits and that the IOP in Kir6.2(−/−) mice can be effectively reduced by LFA.
Determination of CKLP1 Optimal Dose in Rabbits
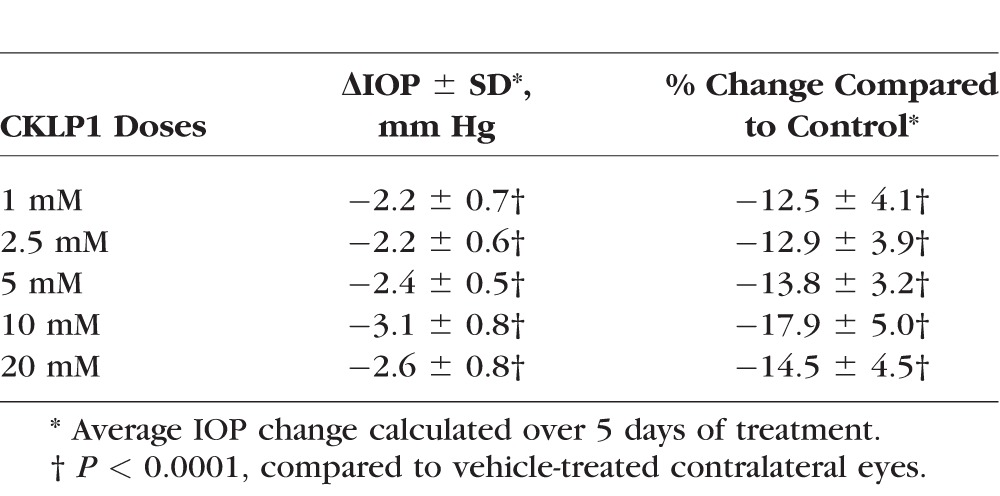
The cornea in mouse is extremely thin in comparison to the human cornea. To address whether CKLP1 permeability may be affected by a thicker cornea, we assessed different doses of CKLP1 in Dutch-belted pigmented rabbits. Although all doses (1, 2.5, 5, 10, and 20 mM) showed significant reduction in IOP as compared to the contralateral vehicle-treated control eyes, the 10-mM dose was found to exhibit maximum ocular hypotensive properties with an absolute IOP reduction of 3.1 ± 0.8 mm Hg (P < 0.0001) with a range of −2.8 ± 0.9 to −3.5 ± 1.0 mm Hg as compared to the control eyes (Table 2). This corresponds to an overall reduction of 17.9% ± 5.0% for the treatment days.
Table 2.
CKLP1 Dose Response in Dutch-Belted Pigmented Rabbits
Effect of Combination Treatment With CKLP1 and Known Ocular Hypotension Agents in Rabbits
From results in mice showing an additive effect of CKLP1 with LFA, and owing to the unique IOP-lowering ability of CKLP1 through modification of episcleral venous pressure, we hypothesized that CKLP1 would work additively to lower IOP with existing ocular hypotensive agents (e.g., latanoprost, timolol and Rho kinase inhibitor Y27632), each with different reported mechanisms of action.53–56
CKLP1 and LFA
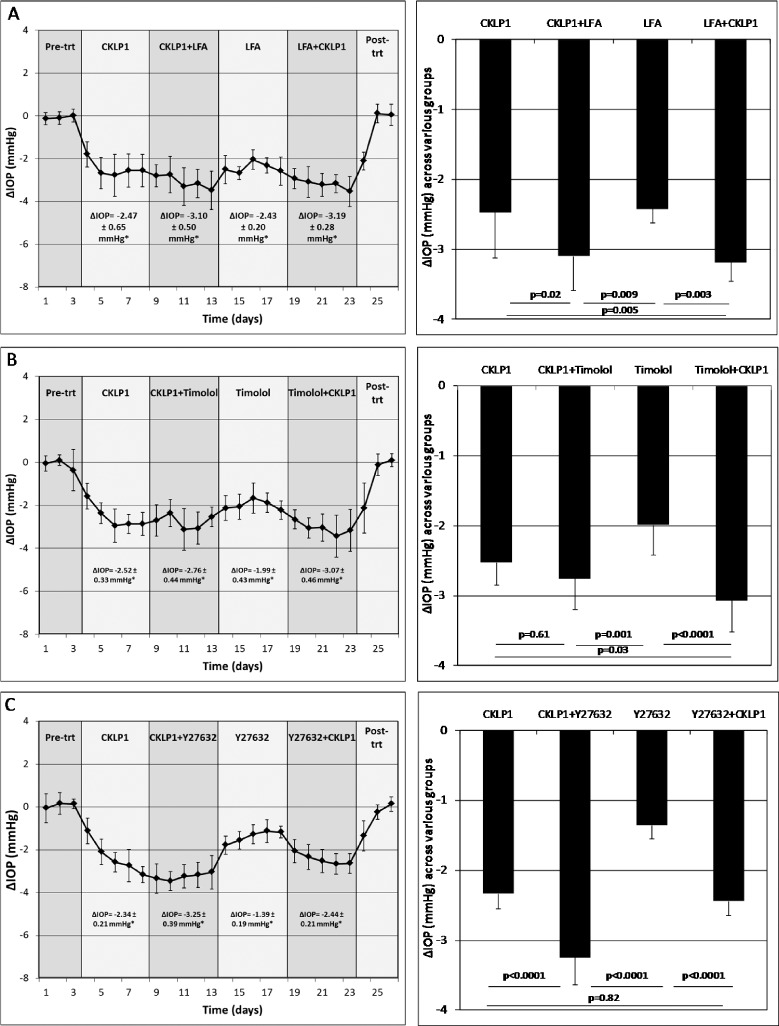
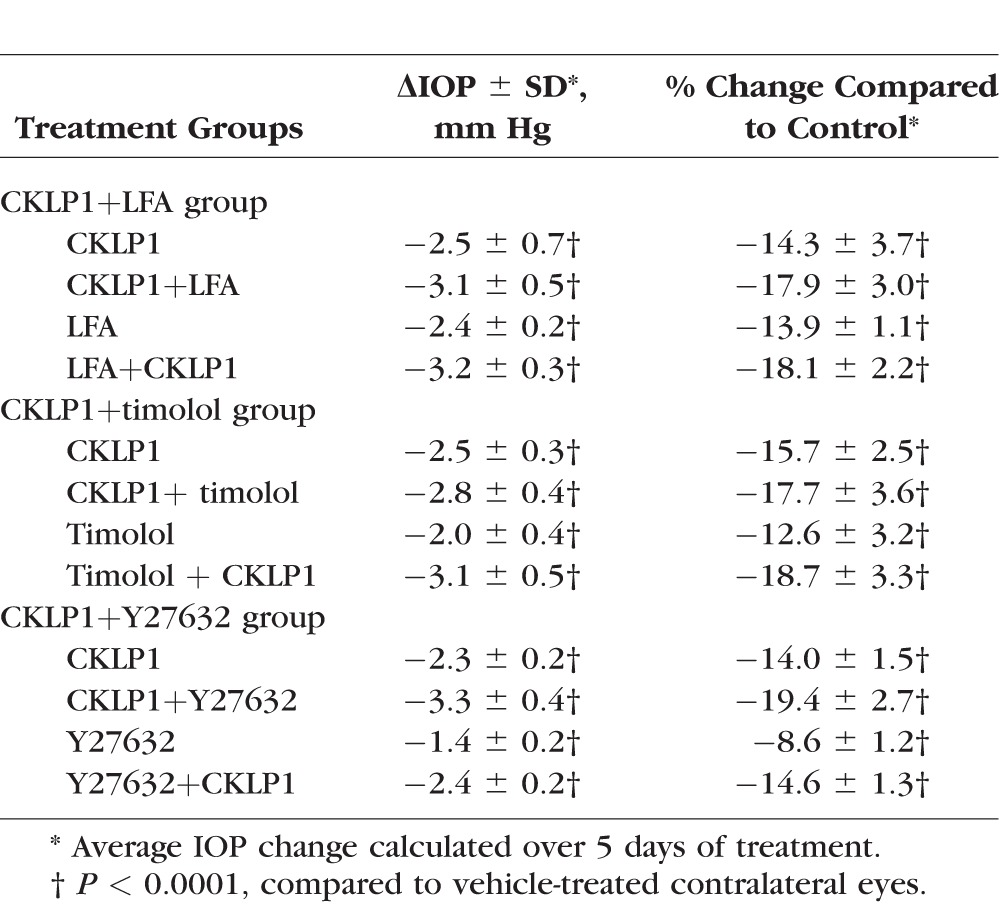
One eye of each Dutch-belted pigmented rabbit (n = 10) was treated with CKLP1 either alone or in combination with LFA. CKLP1+LFA lowered IOP by 3.1 ± 0.5 mm Hg (P < 0.0001) when compared to CKLP1 alone (2.5 ± 0.7 mm Hg, P < 0.0001) and by 3.2 ± 0.3 mm Hg (P < 0.0001) when compared to LFA alone (2.4 ± 0.2 mm Hg, P < 0.0001) (Fig. 4A; Table 3). Combination treatment with CKLP1+LFA resulted in a 38.6% ± 62.3% (P = 0.004) greater reduction than CKLP1 alone and a 30.3% ± 11.1% (P = 0.003) greater reduction than LFA alone. Comparison of absolute IOP reduction indicates that combination treatment with CKLP1+LFA showed significantly more IOP reduction than either CKLP1 or LFA treatment alone.
Figure 4.
Effect of combination treatment with CKLP1 and ocular hypotensive agents. (A) Combination treatment with CKLP1+LFA produced a significant additive increase in IOP reduction when compared to CKLP1 (P < 0.0001) or LFA alone (P < 0.0001). (B) Combination treatment with CKLP1+timolol showed a statistically significant drop in IOP following timolol alone (P < 0.0001) but not following CKLP1 alone (P = 0.61). (C) Combination treatment with CKLP1+Y27632 produced a greater IOP reduction (P < 0.0001) than CKLP1 or Y27632 alone. Values are mean ± standard deviation. Trt, treatment.
Table 3.
The Effect of Various Combination Treatments With CKLP1 and Other Ocular Hypotensive Agents on IOP in Dutch-Belted Pigmented Rabbits
CKLP1 and Timolol
In Dutch-belted pigmented rabbits (n = 10) treated with CKLP1+timolol, IOP was lowered by 2.8 ± 0.4 mm Hg (P < 0.0001) in comparison to CKLP1 alone (2.5 ± 0.3 mm Hg, P < 0.0001) and by 3.1 ± 0.5 mm Hg (P < 0.0001) when compared to timolol alone (2.0 ± 0.4 mm Hg, P < 0.0001) (Fig. 4B; Table 3). Comparison of CKLP1+timolol to CKLP1 showed a modest additive effect of 12.5% ± 18.8% but failed to achieve statistical significance (P = 0.61). When these animals were treated with CKLP1+timolol following timolol-alone treatment, a 52.3% ± 21.9% IOP reduction was achieved (P < 0.0001). The second combination treatment of CKLP1+timolol showed significant additive effect (P = 0.03) when compared to IOP reduction values obtained with CKLP1 alone.
CKLP1 and Y27632
Dutch-belted pigmented rabbits (n = 10) treated with CKLP1+Y27632 caused a mean IOP reduction of 3.3 ± 0.4 mm Hg (P < 0.0001, compared to controls), equivalent to a 38.2% ± 14.8% greater IOP reduction than CKLP1 alone (2.3 ± 0.2 mm Hg, P < 0.0001) (Fig. 4C; Table 3). Likewise, combination treatment with CKLP1+Y27632 lowered IOP by an additional 74.6% ± 28.2% (P < 0.0001) compared to Y27632 alone (1.4 ± 0.2 mm Hg, P < 0.0001).
Discussion
The reduction of IOP to a predetermined target level is the only known glaucoma treatment that can slow disease progression.3–9,38 Although several therapeutic classes of drugs are available as ocular hypotensive agents, their long-term use is often complicated with side effects, inadequate reduction of IOP, and eventual reduction in efficacy after long-term use, warranting the development of newer drugs for future therapeutic management of the disease. CKLP1, a novel water-soluble prodrug of the KATP channel opener cromakalim, has been shown to retain the ocular hypotensive properties of its parent compound in several normotensive animal models.43 In the current study, we showed that CKLP1 exerts its IOP-lowering effect by decreasing episcleral venous pressure, presumably by modifying the distal portion of the conventional outflow pathway. CKLP1 also worked in an additive manner to lower IOP when used in combination with LFA, timolol, and Rho kinase inhibitor Y27632. Together, these results suggest that CKLP1 has a unique mechanism of action from other IOP-lowering agents, which probably accounts for its ability to work in combination with other glaucoma treatment options to lower IOP.
CKLP1 directly affects episcleral venous pressure, while not showing a significantly detectable effect on outflow facility, aqueous humor flow rate, or uveoscleral outflow. Among existing glaucoma drugs, LFA has been shown to have a modest effect on episcleral venous pressure in mice (although not confirmed in other animal species),47 while measurements in humans, using a pressure chamber technique, have shown no change.57,58 However, LFA, unlike CKLP1, lowers IOP by increasing outflow facility, mainly through the uveoscleral pathway.47 Likewise, the Rho kinase inhibitor AR13324 has been found to alter episcleral venous pressure in rabbits,59 but it too has effects on other aspects of aqueous humor dynamics such as aqueous outflow and production of aqueous humor.22 The selective α-adrenergic agonist brimonidine has been shown to lower episcleral venous pressure in rabbit eyes besides reducing aqueous flow, ciliary blood flow, and ciliary oxygen tension. However, in a separate study done in human subjects, brimonidine does not affect the episcleral venous pressure and is only involved in increasing uveoscleral outflow facility,60 although suppression of aqueous humor production through vasoconstriction has been reported as an acute effect of brimonidine.61 Taken together, it is evident that none of the existing glaucoma drugs have a direct and singular effect on episcleral venous pressure. In this respect, the novel finding that CKLP1 specifically targets episcleral venous pressure is unique, compared to existing glaucoma therapeutics.
The action of CKLP1 on the episcleral venous system of the conventional outflow pathway was further validated by experiments performed in the human anterior segment perfusion model. This model only contains the conventional outflow pathway, as the ciliary body is removed to eliminate the uveoscleral outflow pathway, and no blood flow is present, effectively removing episcleral venous pressure. Our results in the human anterior segment culture model where the trabecular meshwork and inner wall and parts of the outer wall of Schlemm's canal were removed suggest that CKLP1 has an effect on the distal outflow pathway. The distal outflow pathway consists of the outer wall of Schlemm's canal, collector channels, and the deep scleral plexus vessels.62 It is believed that the episcleral venous system in conjunction with the distal outflow pathway has a significant effect in the pathophysiology of glaucoma.62,63 For example, increased pulsatile flow of aqueous humor in the episcleral veins, in response to elevated IOP, is lost in glaucoma patients with a direct correlation between the loss of pulsatile flow to the severity of glaucoma.64–67 Although the current dogma suggests that the majority of resistance to aqueous outflow is found at the junction of the trabecular meshwork and Schlemm's canal, several studies have indicated that the distal outflow pathway is a key contributor, accounting for up to 50% of total outflow resistance.62,63,68–71 In the anterior segment culture model, although there is no systemic blood flow, the existing episcleral vessels may still maintain enough resistance to affect the flow of fluids through them. Endothelial and endothelial-like cells line the walls of the episcleral vessels within the distal outflow pathway, but the molecular mechanisms governing the properties and functions of these cells are largely unknown.62 KATP channel openers are well-known modulators of endothelial cell properties and have often been used as vasodilators for lowering systemic blood pressure19,72–75 Therefore, it would not be surprising if CKLP1 has a relaxing effect on endothelial cell–lined episcleral veins, resulting in lower episcleral venous pressure in the distal outflow pathway and ultimately reduced IOP. Further investigations assessing the role of CKLP1 in the distal outflow pathway are warranted to assess the effect on fluid flow through this region.
Intracellular signaling mechanisms of glaucoma drugs are mostly unknown. There is evidence of cytoskeletal modulation by rho kinase inhibitors,76 whereas latanoprost has been shown to increase phosphoinositide turnover following binding with FP receptors in human trabecular meshwork and rat vascular smooth muscle cells.77–79 Additionally, latanoprost can also affect platelet activity factor in rabbit blood80 and endothelin receptors of human choroidal melanocytes.81 A recently published study from our laboratory82 has found that the IOP-lowering effect of latanoprost is mediated by signaling through stanniocalcin-1 (STC-1) and that STC-1 by itself can mimic the ocular hypotensive effect of latanoprost. Work from our laboratory indicates that the Erk1/2 signaling pathway is activated in the cells of the conventional outflow pathway following treatment with KATP channel openers.83 However, it remains to be seen if similar pathways are involved in episcleral venous cells in response to treatment with CKLP1.
In the past, β-blockers were considered the first line of glaucoma drugs84 but were later replaced by prostaglandin analogs during the 1990s, owing to the latter's increased efficacy and fewer systemic side effects.84–86 Unfortunately, monotherapies with front-line glaucoma drugs like latanoprost or timolol are often inadequate in many patients, and thus require addition of more than one drug for effective IOP reduction.5,87 A single daily dose of CKLP1, in combination with drugs from the prostaglandin analog (latanoprost) and the β-blocker family (timolol), as well as the Rho kinase inhibitor Y27632, showed statistically significant additive effects on IOP. The only exception was the initial treatment with CKLP1 followed by CKLP1+timolol. While combination therapy showed a modest additive IOP reduction with CKLP1+timolol in comparison to CKLP1 alone, this was not statistically significant. However, the additive effect was more pronounced and statistically significant when timolol was used first, followed by treatment with timolol+CKLP1. Since CKLP1 does not have a significant effect on inflow, one possible reason for this could be the comparatively high efficacy of both drugs in lowering IOP. Because we used normotensive animals, we may have reached the maximum reduction threshold with these two drugs.
It should be noted that our studies should not be used to compare efficacies of individual drugs. Comparing the IOP-lowering potential between drugs was not the goal of this study. The experimental approach was designed to evaluate whether CKLP1 would work in combination with existing glaucoma drugs. Pending future clinical trials, these data may only be considered as proof of concept for future use of these drug combinations as a fixed-dose formulation for treating glaucoma patients.
One limitation of this study was the use of normotensive animals, in place of animal models with elevated IOP. Rodent models of elevated IOP (e.g., DBA/2J or Col1a1[r/r]) usually work by direct physical blockage of the conventional outflow pathway. Because CKLP1 affects the distal portion of the conventional outflow pathway, it did not seem appropriate to use animal models with a disrupted outflow pathway. It may be noted that using normotensive animals for glaucoma drugs is a common practice, and all known ocular hypotensive agents have been shown to work in animal models with normal IOP.88–92 To further delineate the mechanism of action of CKLP1, it will be necessary to assess the drug's effects in newer animal models, where use of steroids or overexpression of key molecules (e.g., TGFβ2, CTGF) results in elevated IOP.93–96
In summary, the novel KATP channel opener CKLP1 uniquely targets the episcleral venous system and distal outflow pathway, resulting in significant reduction of IOP in normotensive animal models and in ex vivo human eyes. Owing to this unique mode of action, CKLP1 can be successfully used in combination with known ocular hypotensive agents. Taken together, the use of CKLP1, either alone or with other IOP-lowering drugs, may be considered as a potential future therapeutic strategy for clinical management of glaucoma.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health Grants EY21727 (MPF), EY26490 (MPF), and EY022359 (DRO); Minnesota Partnership for Biotechnology and Medical Genomics No. 12.06 (MPF, PID); Minnesota Partnership for Biotechnology and Medical Genomics TPDF No. 15.01 (MPF, PID); National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114 (PID); Fight for Sight (UK) No. 1858 (DRO); Research to Prevent Blindness (URC, TAR, CKB, BHH, MPF); and Mayo Foundation (MPF).
Disclosure: U. Roy Chowdhury, None; T.A. Rinkoski, None; C.K. Bahler, None; J.C. Millar, None; J.A. Bertrand, None; B.H. Holman, None; J.M. Sherwood, None; D.R. Overby, None; K.L. Stoltz, None; P.I. Dosa, None; M.P. Fautsch, None
References
- 1. Quigley HA. . Glaucoma. Lancet. 2011; 377: 1367– 1377. [DOI] [PubMed] [Google Scholar]
- 2. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. . Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121: 2081– 2090. [DOI] [PubMed] [Google Scholar]
- 3. Boland MV, Ervin AM, Friedman D,et al. . Treatment for Glaucoma: Comparative Effectiveness. Rockville, MD: U.S. Department of Health and Human Services; 2012. [Google Scholar]
- 4. Boland MV, Ervin AM, Friedman DS,et al. . Comparative effectiveness of treatments for open-angle glaucoma: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013; 158: 271– 279. [DOI] [PubMed] [Google Scholar]
- 5. Kass MA, Heuer DK, Higginbotham EJ,et al. . The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 701– 713, discussion 829–830. [DOI] [PubMed] [Google Scholar]
- 6. Heijl A, Leske MC, Bengtsson B, Hyman L, Hussein M. . Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120: 1268– 1279. [DOI] [PubMed] [Google Scholar]
- 7. Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. . Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007; 114: 1965– 1972. [DOI] [PubMed] [Google Scholar]
- 8. Leske MC, Heijl A, Hussein M,et al. . Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003; 121: 48– 56. [DOI] [PubMed] [Google Scholar]
- 9. Garway-Heath DF, Crabb DP, Bunce C,et al. . Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015; 385: 1295– 1304. [DOI] [PubMed] [Google Scholar]
- 10. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures: Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998; 126: 487– 497. [DOI] [PubMed] [Google Scholar]
- 11. Cheema A, Chang RT, Shrivastava A, Singh K. . Update on the medical treatment of primary open-angle glaucoma. Asia Pac J Ophthalmol (Phila). 2016; 5: 51– 58. [DOI] [PubMed] [Google Scholar]
- 12. Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S; for the Low-Pressure Glaucoma Study Group . A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011; 151: 671– 681. [DOI] [PubMed] [Google Scholar]
- 13. Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998; 126: 498– 505. [DOI] [PubMed] [Google Scholar]
- 14. Kaufman PL. . Latanoprostene bunod ophthalmic solution 0.024% for IOP lowering in glaucoma and ocular hypertension. Expert Opin Pharmacother. 2017; 18: 433– 444. [DOI] [PubMed] [Google Scholar]
- 15. Roy Chowdhury U, Hann CR, Stamer WD, Fautsch MP. Aqueous humor outflow: dynamics and disease. Invest Ophthalmol Vis Sci. 2015; 56: 2993– 3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmidl D, Schmetterer L, Garhofer G, Popa-Cherecheanu A. . Pharmacotherapy of glaucoma. J Ocul Pharmacol Ther. 2015; 31: 63– 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alm A, Grierson I, Shields MB. . Side effects associated with prostaglandin analog therapy. Surv Ophthalmol. 2008; 53 suppl 1: S93– S105. [DOI] [PubMed] [Google Scholar]
- 18. Alm A, Stjernschantz J;. for the Scandinavian Latanoprost Study Group. Effects on intraocular pressure and side effects of 0.005% latanoprost applied once daily, evening or morning: a comparison with timolol. Ophthalmology. 1995; 102: 1743– 1752. [DOI] [PubMed] [Google Scholar]
- 19. Roy Chowdhury U, Dosa PI, Fautsch MP. ATP sensitive potassium channel openers: a new class of ocular hypotensive agents. Exp Eye Res. 2017; 158: 85– 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Germano RA, Finzi S, Challa P, Susanna R. Junior. Rho kinase inhibitors for glaucoma treatment: review. Arq Bras Oftalmol. 2015; 78: 388– 391. [DOI] [PubMed] [Google Scholar]
- 21. Lu LJ, Tsai JC, Liu J. . Novel pharmacologic candidates for treatment of primary open-angle glaucoma. Yale J Biol Med. 2017; 90: 111– 118. [PMC free article] [PubMed] [Google Scholar]
- 22. Wang RF, Williamson JE, Kopczynski C, Serle JB. . Effect of 0.04% AR-13324, a ROCK, and norepinephrine transporter inhibitor, on aqueous humor dynamics in normotensive monkey eyes. J Glaucoma. 2015; 24: 51– 54. [DOI] [PubMed] [Google Scholar]
- 23. Zhong Y, Yang Z, Huang WC, Luo X. . Adenosine, adenosine receptors and glaucoma: an updated overview. Biochim Biophys Acta. 2013; 1830: 2882– 2890. [DOI] [PubMed] [Google Scholar]
- 24. Williams RD, Novack GD, van Haarlem T, Kopczynski C. . Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am J Ophthalmol. 2011; 152: 834– 841.e1. [DOI] [PubMed] [Google Scholar]
- 25. Harris A, Ward CL, Rowe-Rendleman CL,et al. . Ocular hypotensive effect of ONO-9054, an EP3/FP receptor agonist: results of a randomized, placebo-controlled, dose escalation study. J Glaucoma. 2016; 25: e826– e833. [DOI] [PubMed] [Google Scholar]
- 26. Lewis RA, Levy B, Ramirez N,et al. . Fixed-dose combination of AR-13324 and latanoprost: a double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br J Ophthalmol. 2016; 100: 339– 344. [DOI] [PubMed] [Google Scholar]
- 27. Miller Ellis E, Berlin MS, Ward CL, Sharpe JA, Jamil A, Harris A. Ocular hypotensive effect of the novel EP3/FP agonist ONO-9054 versus Xalatan: results of a 28-day, double-masked, randomised study. Br J Ophthalmol. 2016; 101: 796– 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Myers JS, Sall KN, DuBiner H,et al. . A dose-escalation study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of 2 and 4 weeks of twice-daily ocular trabodenoson in adults with ocular hypertension or primary open-angle glaucoma. J Ocul Pharmacol Ther. 2016; 32: 555– 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suto F, Rowe-Rendleman CL, Ouchi T, Jamil A, Wood A, Ward CL. . A novel dual agonist of EP3 and FP receptors for OAG and OHT: safety, pharmacokinetics, and pharmacodynamics of ONO-9054 in healthy volunteers. Invest Ophthalmol Vis Sci. 2015; 56: 7963– 7970. [DOI] [PubMed] [Google Scholar]
- 30. Tanihara H, Inoue T, Yamamoto T,et al. . Intra-ocular pressure-lowering effects of a Rho kinase inhibitor, ripasudil (K-115), over 24 hours in primary open-angle glaucoma and ocular hypertension: a randomized, open-label, crossover study. Acta Ophthalmol. 2015; 93: e254– e260. [DOI] [PubMed] [Google Scholar]
- 31. Tanihara H, Inoue T, Yamamoto T,et al. . Additive intraocular pressure-lowering effects of the rho kinase inhibitor ripasudil (K-115) combined with timolol or latanoprost: a report of 2 randomized clinical trials. JAMA Ophthalmol. 2015; 133: 755– 761. [DOI] [PubMed] [Google Scholar]
- 32. Weinreb RN, Ong T, Scassellati Sforzolini B,et al. . A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol. 2015; 99: 738– 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weinreb RN, Scassellati Sforzolini B, Vittitow J, Liebmann J. Latanoprostene bunod 0.024% versus timolol maleate 0.5% in subjects with open-angle glaucoma or ocular hypertension: The APOLLO Study. Ophthalmology. 2016; 123: 965– 973. [DOI] [PubMed] [Google Scholar]
- 34. Tanihara H, Inatani M, Honjo M, Tokushige H, Azuma J, Araie M. . Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch Ophthalmol. 2008; 126: 309– 315. [DOI] [PubMed] [Google Scholar]
- 35. Toris CB, McLaughlin MA, Dworak DP,et al. . Effects of Rho kinase inhibitors on intraocular pressure and aqueous humor dynamics in nonhuman primates and rabbits. J Ocul Pharmacol Ther. 2016; 32: 355– 364. [DOI] [PubMed] [Google Scholar]
- 36. Honjo M, Tanihara H, Inatani M,et al. . Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001; 42: 137– 144. [PubMed] [Google Scholar]
- 37. Garcia GA, Ngai P, Mosaed S, Lin KY. . Critical evaluation of latanoprostene bunod in the treatment of glaucoma. Clin Ophthalmol. 2016; 10: 2035– 2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Conlon R, Saheb H, . Ahmed II. Glaucoma treatment trends: a review. Can J Ophthalmol. 2017; 52: 114– 124. [DOI] [PubMed] [Google Scholar]
- 39. Rasmussen CA, Kaufman PL. . Exciting directions in glaucoma. Can J Ophthalmol. 2014; 49: 534– 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roy Chowdhury U, Bahler CK, Holman BH, Dosa PI, Fautsch MP. Ocular hypotensive effects of the ATP-sensitive potassium channel opener cromakalim in human and murine experimental model systems. PLoS One. 2015; 10: e0141783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chowdhury UR, Holman BH, Fautsch MP. . ATP-sensitive potassium (KATP) channel openers diazoxide and nicorandil lower intraocular pressure in vivo. Invest Ophthalmol Vis Sci. 2013; 54: 4892– 4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chowdhury UR, Bahler CK, Hann CR,et al. . ATP-sensitive potassium (KATP) channel activation decreases intraocular pressure in the anterior chamber of the eye. Invest Ophthalmol Vis Sci. 2011; 52: 6435– 6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roy Chowdhury U, Viker KB, Stoltz KL, Holman BH, Fautsch MP, Dosa PI. Analogs of the ATP-sensitive potassium (KATP) channel opener cromakalim with in vivo ocular hypotensive activity. J Med Chem. 2016; 59: 6221– 6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sherwood JM, Reina-Torres E, Bertrand JA, Rowe B, Overby DR. . Measurement of outflow facility using iPerfusion. PLoS One. 2016; 11: e0150694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reina-Torres E, Wen JC, Liu KC,et al. . VEGF as a paracrine regulator of conventional outflow facility. Invest Ophthalmol Vis Sci. 2017; 58: 1899– 1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Millar JC, Phan TN, Pang IH, Clark AF. . Strain and age effects on aqueous humor dynamics in the mouse. Invest Ophthalmol Vis Sci. 2015; 56: 5764– 5776. [DOI] [PubMed] [Google Scholar]
- 47. Millar JC, Clark AF, Pang IH. . Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Invest Ophthalmol Vis Sci. 2011; 52: 685– 694. [DOI] [PubMed] [Google Scholar]
- 48. Bahler CK, Hann CR, Fjield T, Haffner D, Heitzmann H, Fautsch MP. . Second-generation trabecular meshwork bypass stent (iStent inject) increases outflow facility in cultured human anterior segments. Am J Ophthalmol. 2012; 153: 1206– 1213. [DOI] [PubMed] [Google Scholar]
- 49. Sjoquist B, Stjernschantz J. . Ocular and systemic pharmacokinetics of latanoprost in humans. Surv Ophthalmol. 2002; 47 suppl 1: S6– S12. [DOI] [PubMed] [Google Scholar]
- 50. Calissendorff B, Sjoquist B, Hogberg G, Grunge-Lowerud A. . Bioavailability in the human eye of a fixed combination of latanoprost and timolol compared to monotherapy. J Ocul Pharmacol Ther. 2002; 18: 127– 131. [DOI] [PubMed] [Google Scholar]
- 51. Nakagawa H, Yoshioka K, Miyahara E, Fukushima Y, Tamura M, Itoh K. . Intrathecal administration of Y-27632, a specific rho-associated kinase inhibitor, for rat neoplastic meningitis. Mol Cancer Res. 2005; 3: 425– 433. [DOI] [PubMed] [Google Scholar]
- 52. Ishizaki T, Uehata M, Tamechika I,et al. . Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000; 57: 976– 983. [PubMed] [Google Scholar]
- 53. Toris CB, Gabelt BT, Kaufman PL. . Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. 2008; 53 suppl 1: S107– S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schehlein EM, Novack GD, Robin AL. . New classes of glaucoma medications. Curr Opin Ophthalmol. 2017; 28: 161– 168. [DOI] [PubMed] [Google Scholar]
- 55. Curran MP, Orman JS. . Bimatoprost/timolol: a review of its use in glaucoma and ocular hypertension. Drugs Aging. 2009; 26: 169– 184. [DOI] [PubMed] [Google Scholar]
- 56. Challa P, Arnold JJ. . Rho-kinase inhibitors offer a new approach in the treatment of glaucoma. Expert Opin Investig Drugs. 2014; 23: 81– 95. [DOI] [PubMed] [Google Scholar]
- 57. Sponsel WE, Mensah J, Kiel JW,et al. . Effects of latanoprost and timolol-XE on hydrodynamics in the normal eye. Am J Ophthalmol. 2000; 130: 151– 159. [DOI] [PubMed] [Google Scholar]
- 58. Sit AJ, McLaren JW. . Measurement of episcleral venous pressure. Exp Eye Res. 2011; 93: 291– 298. [DOI] [PubMed] [Google Scholar]
- 59. Kiel JW, Kopczynski CC. . Effect of AR-13324 on episcleral venous pressure in Dutch belted rabbits. J Ocul Pharmacol Ther. 2015; 31: 146– 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fan S, Agrawal A, Gulati V, Neely DG, Toris CB. . Daytime and nighttime effects of brimonidine on IOP and aqueous humor dynamics in participants with ocular hypertension. J Glaucoma. 2014; 23: 276– 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Toris CB, Camras CB, Yablonski ME. . Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Am J Ophthalmol. 1999; 128: 8– 14. [DOI] [PubMed] [Google Scholar]
- 62. Carreon T, van der Merwe E, Fellman RL, Johnstone M, Bhattacharya SK. . Aqueous outflow: a continuum from trabecular meshwork to episcleral veins. Prog Retin Eye Res. 2017; 57: 108– 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Overby DR, Stamer WD, Johnson M. . The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp Eye Res. 2009; 88: 656– 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ascher KW. . The Aqueous Veins: Biomicroscopic Study of Aqueous Humor Elimination. Springfield, Illinois: Charles C. Thomas; 1961. [Google Scholar]
- 65. Johnstone M, Martin E, Jamil A. . Pulsatile flow into the aqueous veins: manifestations in normal and glaucomatous eyes. Exp Eye Res. 2011; 92: 318– 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kleinert HW. . The compensation maximum: a new glaucoma sign in aqueous veins. AMA Arch Ophthalmol. 1951; 46: 618– 624. [PubMed] [Google Scholar]
- 67. Stambaugh JL, Fuhs JC, Ascher KW. . Study of the compensation-maximum test on aqueous veins. AMA Arch Ophthalmol. 1954; 51: 24– 31. [DOI] [PubMed] [Google Scholar]
- 68. Maepea O, Bill A. . Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res. 1992; 54: 879– 883. [DOI] [PubMed] [Google Scholar]
- 69. Minckler DS, Baerveldt G, Alfaro MR, Francis BA. . Clinical results with the Trabectome for treatment of open-angle glaucoma. Ophthalmology. 2005; 112: 962– 967. [DOI] [PubMed] [Google Scholar]
- 70. Van Buskirk EM. . Trabeculotomy in the immature, enucleated human eye. Invest Ophthalmol Vis Sci. 1977; 16: 63– 66. [PubMed] [Google Scholar]
- 71. Grant WM. . Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963; 69: 783– 801. [DOI] [PubMed] [Google Scholar]
- 72. Gribble FM, Reimann F. . Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003; 46: 875– 891. [DOI] [PubMed] [Google Scholar]
- 73. Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. . Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010; 90: 291– 366. [DOI] [PubMed] [Google Scholar]
- 74. Rodrigo GC, Standen NB. . ATP-sensitive potassium channels. Curr Pharm Des. 2005; 11: 1915– 1940. [DOI] [PubMed] [Google Scholar]
- 75. Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. . Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989; 245: 177– 180. [DOI] [PubMed] [Google Scholar]
- 76. Inoue T, Tanihara H. . Rho-associated kinase inhibitors: a novel glaucoma therapy. Prog Retin Eye Res. 2013; 37: 1– 12. [DOI] [PubMed] [Google Scholar]
- 77. Sharif NA, Kelly CR, Crider JY. . Human trabecular meshwork cell responses induced by bimatoprost, travoprost, unoprostone, and other FP prostaglandin receptor agonist analogues. Invest Ophthalmol Vis Sci. 2003; 44: 715– 721. [DOI] [PubMed] [Google Scholar]
- 78. Griffin BW, Magnino PE, Pang IH, Sharif NA. . Pharmacological characterization of an FP prostaglandin receptor on rat vascular smooth muscle cells (A7r5) coupled to phosphoinositide turnover and intracellular calcium mobilization. J Pharmacol Exp Ther. 1998; 286: 411– 418. [PubMed] [Google Scholar]
- 79. Ansari HR, Davis AM, Kaddour-Djebbar I, Abdel-Latif AA. . Effects of prostaglandin F2alpha and latanoprost on phosphoinositide turnover, myosin light chain phosphorylation and contraction in cat iris sphincter. J Ocul Pharmacol Ther. 2003; 19: 217– 231. [DOI] [PubMed] [Google Scholar]
- 80. Moschos MM, Nitoda E, Chatziralli IP, Panos GD, Demopoulos CA. . Impact of prostaglandin glaucoma drops on platelet-activating factor action: an in vitro study. Drug Des Devel Ther. 2016; 10: 3977– 3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sharif NA, Crider JY. . Human choroidal melanocyte signal transduction responses to various pharmacological agents: focus on endothelin receptors. Curr Eye Res. 2011; 36: 462– 468. [DOI] [PubMed] [Google Scholar]
- 82. Roddy GW, Viker KB, Winkler NS,et al. . Stanniocalcin-1 is an ocular hypotensive agent and a downstream effector molecule that is necessary for the intraocular pressure-lowering effects of latanoprost. Invest Ophthalmol Vis Sci. 2017; 58: 2715– 2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Roy Chowdhury U, Bahler CK, Holman BH, Fautsch MP. ATP-sensitive potassium (KATP) channel openers diazoxide and nicorandil lower intraocular pressure by activating the Erk1/2 signaling pathway. PLoS One. 2017; 12: e0179345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Realini T. . A history of glaucoma pharmacology. Optom Vis Sci. 2011; 88: 36– 38. [DOI] [PubMed] [Google Scholar]
- 85. Hedman K, Alm A. . A pooled-data analysis of three randomized, double-masked, six-month clinical studies comparing the intraocular pressure reducing effect of latanoprost and timolol. Eur J Ophthalmol. 2000; 10: 95– 104. [DOI] [PubMed] [Google Scholar]
- 86. Hedman K, Alm A, Gross RL. . Pooled-data analysis of three randomized, double-masked, six-month studies comparing intraocular pressure-reducing effects of latanoprost and timolol in patients with ocular hypertension. J Glaucoma. 2003; 12: 463– 465. [DOI] [PubMed] [Google Scholar]
- 87. Musch DC, Lichter PR, Guire KE, Standardi CL. . The Collaborative Initial Glaucoma Treatment Study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999; 106: 653– 662. [DOI] [PubMed] [Google Scholar]
- 88. Akaishi T, Odani-Kawabata N, Ishida N, Nakamura M. . Ocular hypotensive effects of anti-glaucoma agents in mice. J Ocul Pharmacol Ther. 2009; 25: 401– 408. [DOI] [PubMed] [Google Scholar]
- 89. Bito LZ, Draga A, Blanco J, Camras CB. . Long-term maintenance of reduced intraocular pressure by daily or twice daily topical application of prostaglandins to cat or rhesus monkey eyes. Invest Ophthalmol Vis Sci. 1983; 24: 312– 319. [PubMed] [Google Scholar]
- 90. Ota T, Murata H, Sugimoto E, Aihara M, Araie M. . Prostaglandin analogues and mouse intraocular pressure: effects of tafluprost, latanoprost, travoprost, and unoprostone, considering 24-hour variation. Invest Ophthalmol Vis Sci. 2005; 46: 2006– 2011. [DOI] [PubMed] [Google Scholar]
- 91. Zhang H, Yang D, Ross CM, Wigg JP, Pandav S, Crowston JG. . Validation of rebound tonometry for intraocular pressure measurement in the rabbit. Exp Eye Res. 2014; 121: 86– 93. [DOI] [PubMed] [Google Scholar]
- 92. Bar-Ilan A. . The effects of separate and combined topical treatment with timolol maleate and trifluormethazolamide on the intraocular pressure in normal rabbits. Curr Eye Res. 1984; 3: 1305– 1312. [DOI] [PubMed] [Google Scholar]
- 93. Overby DR, Bertrand J, Tektas OY,et al. . Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Invest Ophthalmol Vis Sci. 2014; 55: 4922– 4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Overby DR, Clark AF. . Animal models of glucocorticoid-induced glaucoma. Exp Eye Res. 2015; 141: 15– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF. . Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010; 51: 2067– 2076. [DOI] [PubMed] [Google Scholar]
- 96. Pang IH, Clark AF. . Rodent models for glaucoma retinopathy and optic neuropathy. J Glaucoma. 2007; 16: 483– 505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.