Abstract
Introduction
Nephrotoxicity from drugs accounts for 18% to 27% of cases of acute kidney injury. Determining a genetic predisposition may potentially be important in minimizing risk. The aims of this study are as follows: to determine whether a genetic predisposition exists for the development of drug-induced kidney disease (DIKD), using genome-wide association and whole-genome sequencing studies; to describe the frequency, course, risk factors, resolution and outcomes of DIKD cases; to investigate the role of ethnic/racial variability in the genetics of DIKD; and to explore the use of different tools establishing causality of DIKD.
Methods
A total of 800 patients will be enrolled worldwide and blood samples for DNA collected. Data on the patient risk factors, vital signs, laboratory parameters, drug exposure, and DIKD course will be recorded. A panel of nephrologists will adjudicate all cases. Genome-wide association studies will be conducted using population controls matched on biogeographic ancestry to determine whether there is a genetic predisposition to DIKD. The primary endpoint is the identification of specific drug-related polymorphisms associated with DIKD. Secondary endpoints include the following: frequency of DIKD by causal drug and drug combinations; DIKD genetic variability; exploration of causality assessment tools; risk factor identification; description of the course of DIKD, including mortality and dialysis dependency at hospital discharge and 28 and 90 days post-event.
Results
Data are currently being analyzed. Results are pending.
Discussion
The Genetic Contribution to Drug Induced Renal Injury (DIRECT) study will be the first observational cohort study to investigate the genetic determinants of DIKD. If the trial is positive, its findings will potentially translate into safer patient outcomes, by genotypic individualization of therapy and minimization of harm.
Keywords: AKI, antimicrobials, calcineurin inhibitors, nephrotoxicity, NSAIDs, pharmacogenomics
Drug-induced kidney disease (DIKD) is a common cause of acute kidney injury (AKI) in ambulatory and hospitalized patients. The phenotype of DIKD is variable, as injury can occur in different structures of the kidney including the vascular endothelium, glomeruli, tubules, and interstitium. In addition, DIKD can manifest as acute and/or chronic alteration of kidney function with onset varying from hours to weeks. It is often asymptomatic, and the diagnosis is based on a biomarker change such as an increased serum creatinine (Scr) or urinary findings including proteinuria and hematuria consistent with glomerular injury. Common drug culprits include antimicrobials, calcineurin inhibitors and chemotherapeutic agents. Risk factors for DIKD have been reported for individual drugs; they can be patient-specific (e.g., age, chronic kidney disease [CKD]), disease related (e.g., sepsis, volume depletion), and process of care related (e.g., drug dose and duration). Most cases of DIKD resolve with drug discontinuation or dose reduction. However, it is recognized that recurrent or prolonged cases of AKI may lead to the development or progression of CKD. Effective strategies to predict DIKD may help to reduce the risk of recurrent injuries.
The pharmacogenomics of DIKD remains to be established. Similar to adverse drug reactions affecting other organs, it is most likely that there will be interplay between a number of different genes and environmental factors. The genes involved may affect diverse cellular pathways, including drug metabolism and transport, apoptosis, immune responses, and cellular repair and regeneration. Several drugs interact with organic anion transporters (OAT) and may cause kidney injury due to intracellular accumulation caused by alterations in OAT function.1 The genotype of 2 transporters, OCT2 and multi-drug and toxin extrusion protein (MATE), contribute to the susceptibility of cisplatin nephrotoxicity.2 Similar mechanisms could operate for other drugs. The immune response in drug-induced acute interstitial nephritis (AIN) has not been well studied. Genetic polymorphisms in human leukocyte antigens (HLAs) have been documented in antibiotic-associated hepatotoxicity and may similarly have a role in drug-induced AIN.3, 4
Genomic approaches such as genome-wide association studies (GWAS) and whole-genome sequencing enable the detection of rare, serious adverse effects, provided that a well-defined, reliable phenotype is established. Given the recent success in identifying genes associated with other adverse drug reactions, including the liver,5 skin,6 and muscle injury,7 a similar study to identify genetic factors relevant to the risk of DIKD is timely. This would be helpful to personalize drug treatment and to inform drug development processes where nephrotoxicity limits the generation of new drugs. The Genetic Contribution to Drug Induced Renal Injury (DIRECT) study is an observational genomic study of patients who have developed DIKD to determine the genetic predictors of DIKD occurring in adult and pediatric patients from an international consortium of investigators.
Study Objectives
The primary objective of the study is to identify common polymorphisms in subjects with DIKD compared to population-based controls using genome-wide association analysis. Secondary objectives include the following: to investigate the role of ethnic/racial variability in the genetics of DIKD associated with specific, high-volume drugs; to describe the course, clinical risk factors, resolution, and outcomes of DIKD; and to explore the utility of different causality assessment tools when adjudicating cases of DIKD.
Study Design
This study is an international, multi-center, observational cohort study of patients who have developed DIKD as defined by phenotype standardization.8 Drug-induced kidney disease is a spectrum of injury that often goes unrecognized. Phenotype standardization allows for definition of the injury with the aim of improving identification across a variety of clinical settings.8 A panel of nephrologists, adult and pediatric, and pharmacists convened to standardize the phenotype of DIKD for the purpose of inclusion into this study; the phenotype is summarized in Table 1.8 The DIRECT study will primarily focus on the AKI and glomerular phenotypes, as it was believed that tubular disorders and nephrolithiasis would be more difficult to link to genetic polymorphisms. In addition, the study will assess multi-drug injury, allowing up to 3 causal drugs, as the clinical spectrum of DIKD often includes more than 1 causal agent.
Table 1.
Primary and secondary criteria for individual phenotypes
| Phenotype | Acute kidney injury | Glomerular disorder | Nephrolithiasis | Tubular dysfunction |
|---|---|---|---|---|
| Characteristics |
|
|
|
|
| Primary criteria |
|
|
|
Tubular: Hypo-phosphatemia
|
| Secondary criteria |
|
|
|
Phosphaturia
|
AIN, acute interstitial nephritis; ATN, acute tubular necrosis; DM, diabetes mellitus; FeNa, fractional excretion of sodium; FePO4, fractional excretion of phosphorus; GN, glomerulonephritis; HPF, high-powered field; LDH, lactate dehydrogenase; RBC, red blood cell; SIADH, syndrome of inappropriate antidiuretic hormone; UACR, urine albumin to creatinine ratio; UPC, urine protein to creatinine ratio; WBC, white blood cell.8
Hemodynamic changes may contribute to ATN, however, in the absence of any specific features are not considered individual criteria for the AKI phenotype.
SIADH does not reflect direct tubular damage but rather the impact of a drug on ADH secretion and subsequent impaired water handling.
For the primary endpoint, population-based controls matched on ancestry will be used from the Population Reference Sample (POPRES) database.9 Population-based controls have been effectively used in previous genome-wide association studies to examine the genetic basis of serious, rare adverse events such as drug-induced liver injury or skin hypersensitivity.5, 6, 10 Drug-exposed controls allow greater account of possible covariates in the development of the adverse reaction. However, it is often impractical to perform prospective studies following up drug-exposed patients for the development of nephrotoxicity, given the low occurrence of this adverse event and the resources required to study multiple drugs.
At inclusion into the study, subjects will have already experienced kidney injury. Study time points will be based on historical drug exposure and course of injury, including baseline assessment, hospital admission (for inpatients), pre-drug exposure, start of drug exposure, DIKD day, peak serum creatinine (Scr), drug discontinuation or dosage adjustment, nadir Scr, hospital discharge (for inpatients), 28 and 90 days postinjury (Tables 2 and 3). These time points will enhance causality assessment and inform on outcomes. Specifically, the time points were chosen for the following reasons: to establish temporal association between the drug exposure and the injury; to determine the maximal severity of injury; and to measure outcomes of recovery including complete and partial resolution and nonrecovery. Determination of AKI recovery has been variably reported in the literature. For example, resolution has been reported by the nadir Scr time point or at hospital discharge as well as days 28 and 90. We will use definitions proposed by the KDIGO guidelines, determination of acute kidney disease at day 28 and chronic kidney disease at day 90 postinjury. Studies have demonstrated that nonrecovery from AKI is associated with increased mortality.11, 12
Table 2.
Schedule of assessments: Hospitalized subjects
| Variable | Hospital day 1 | Pre−drug exposure | Day of drug exposure | Day of DIKI | Peak Scr or peak severity of injury | Drug DC or dosage adjustment | Nadir Scr or resolution of eventa | Hospital DC | Status at days 28 and 90 |
|---|---|---|---|---|---|---|---|---|---|
| Demographics | X | ||||||||
| Primary criterionb | X | X | X | X | X | X | X | ||
| Etiology of DIKI | X | X | |||||||
| Duration of DIKI | X | X | |||||||
| Risk factors for DIKI | X | X | X | X | X | X | X | X | |
| Drug dosing and concentrations | X | X | X | X | X | X | |||
| Concomitant drugs | X | X | X | X | X | X | X | X | |
| History and physical examination | X | X | X | X | X | X | X | X | |
| Hemodynamics and fluid balance | X | X | X | X | X | X | X | X | |
| Other organ involvement | X | X | X | X | X | X | X | X | |
| SOFA scorec | X | X | X | X | X | X | X | X | |
| Sepsis scorec | X | X | X | X | X | X | X | ||
| Laboratory and imaging data | X | X | X | X | X | X | X | X | |
| Assessment of renal function | X | X | X | X | X | X | X | X | X |
| Blood for DNA and biomarkersd | X | ||||||||
| Urine for biomarkersd | X | ||||||||
| Kidney biopsy if done | X | ||||||||
| Dialysis requirements | X | X | X | X | X | X | |||
| Hospital LOS | X | ||||||||
| Survival status | X | X |
DC, discontinuation; DIKD, drug-induced kidney injury; LOS, length of stay; Scr, serum creatinine; SOFA, sequential organ failure assessment.
If no resolution, data at day 14.
Capture reference and other elements.
Computed.
If feasible; otherwise can be at any time point when consent is obtained.
Table 3.
Schedule of assessments: Ambulatory subjects
| Variable | Pre-drug Exposure | Drug exposure | Day of DIKI | Peak Scr or Peak severity of injury | Drug DC or dosage adjustment | Nadir Scr or Resolution of event |
|---|---|---|---|---|---|---|
| Demographics | X | |||||
| Primary criteriona | X | X | X | X | X | |
| Etiology of DIKI | X | X | ||||
| Duration of DIKI | X | |||||
| Drug dosing history and concentrations | X | X | X | X | X | |
| History and physical examination | X | X | X | X | X | |
| Laboratory and imaging assessment | X | X | X | X | X | X |
| Assessment of renal function | X | X | X | X | X | X |
| Concomitant drugs | X | X | X | X | X | X |
| Blood for DNAb | X | |||||
| Dialysis requirements | X | X | X | X | ||
| Survival status | X |
DC, discontinuation; DIKD, drug-induced kidney injury; Scr, serum creatinine.
Capture reference and other elements.
If feasible, otherwise can be at any time point when consent is obtained.
The following medications or medication classes will be included in the study: antivirals; anti-retrovirals; aminoglycosides; amphotericin; cephalosporins; chemotherapeutic agents; colistin; calcineurin inhibitors; hydralazine; nonsteroidal anti-inflammatory drugs; pamidronate; penicillins; propylthiouracil; proton pump inhibitors; quinolones; rifampin; sulfamethoxazole/trimethoprim; vancomycin; and additional medications to be added as identified.
Study Population
Previously identified or new cases of DIKD will be recruited, as conducting prospective studies on drug-exposed patients to follow them up for the development of nephrotoxicity would be impractical given the low incidence of reactions reported for some drugs. This current approach allows enrollment of a greater number of case patients who have experienced toxicity from a spectrum of drugs.
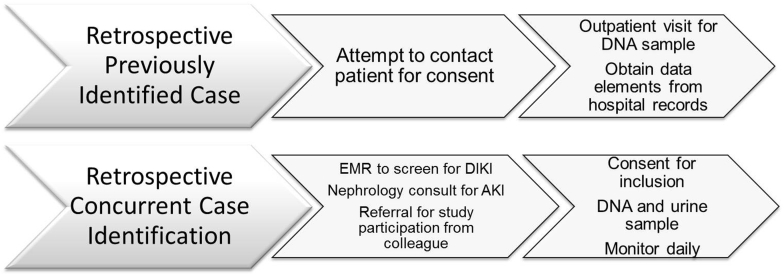
Subjects who have developed DIKD will be identified by investigators and recruited from hospitals or ambulatory care clinics through 2 main approaches (Figure 1):
-
1.
Recall and review of medical records of discharged patients who had DIKD: Patients who developed DIKD previously will be identified through recall, a review of kidney biopsy logs, or previous nephrology consults. They will be contacted for participation, and informed consent will be obtained.
-
2.
Concurrent identification of patients under active treatment: Patients in the hospital or in ambulatory care clinics, who developed DIKD as defined by the primary and secondary criteria in Table 2, will be recruited for the study. They will be identified through screening electronic medical records (EMR) when available. In the absence of an EMR, nephrologists will identify potential subjects from their consult service or from referral from a colleague. If deemed appropriate by their primary physicians, identified case patients will be approached for participation and consent.
Figure 1.
Screening approach for the Genetic Contribution to Drug Induced Renal Injury (DIRECT) study. This figure demonstrates the 2 main approaches to screening subjects who have developed drug-induced kidney injury. The first approach is direct recall of cases in which the investigators have consulted or provided clinical care. The second approach uses electronic surveillance of case patients who have recently developed drug-induced injury and are currently being hospitalized or receiving care at an outpatient clinic.
Inclusion criteria include all patients aged ≥2 years who are exposed to a candidate drug for at least 24 hours and develop the AKI or glomerular injury as defined by the primary criteria for these phenotypes (Table 2).8
Exclusion criteria are as follows: history of or current kidney transplant recipient; history of or current stem cell transplant recipient; CKD stage 5; patient receiving more than 3 causal drugs as determined by the investigators; and incomplete patient information on the time course of drug exposure.
Patients will be screened electronically for inclusion into the study using electronic screening in a Web-based database, www.obriendata.org/direct (last accessed June 2016). Electronic screening will ensure that phenotype criteria from Table 2 are met in relation to the time frame for drug exposure (Supplementary Appendix S1). Reasons for screen and consent failure are recorded. Electronic screening allows for tracking the screened population and most common reasons for screen failure. In addition, this strategy allows for determination of consenting rates.
This study protocol was approved by the UC San Diego Human Research Protection Program (reference #121651). Participating centers will obtain ethics approval through their local ethics committees. All patients will be asked to give their written informed consent to participate in the study. Surrogate consent will be requested if a subject lacks the capacity to provide consent. Participating centers will follow their local ethics committee regulations for consenting procedures. This study was registered as clinical trial number NCT02159209 at www.ClinicalTrials.gov (last accessed June 2016).
Data Collection
Data will be entered into a Web-based database, www.obriendata.org/direct (last accessed June 2016). Data validity and integrity is ensured by electronic rules such as maximum/minimum value checking. Alerts are issued on data in question, and users are required to verify accuracy of data. At baseline, demographics including age, sex, height, weight, self-reported race/ethnicity, and medical history will be obtained from the medical record, including co-morbidities, reason for hospital admission or clinic appointment, nephrology consultation notes, renal biopsy findings and previous surgeries or procedures. The following data will be collected at all time points (Supplementary Appendix S1):
-
1.
Physical examination: The presence of heart abnormalities, peripheral or pulmonary edema, ascites, jaundice, indwelling bladder catheter, as well as any active infections will be recorded.
-
2.
AKI risk factors: Risk factors for AKI will be recorded, including the following: exposure to contrast agents, surgical procedures, need for blood transfusion, nephrotoxic exposures, hypotension (systolic blood pressure < 90 mm Hg or mean arterial pressure < 65 mm Hg), hyperglycemia (blood sugar > 110 mg/dl), intravascular fluid losses (burns, hypovolemia, hemorrhage, paracentesis, or diuresis), sepsis, cardiac failure, and liver disease.
-
3.
Vital signs: Height, weight, blood pressure, temperature, respiratory rate, fluid balance, and intake/output will be recorded. If the patient is in the intensive care unit, we will record the following (if available): central venous pressure, cardiac output, fraction of inspired oxygen, arterial blood gases, and Glasgow Coma Scale score.
-
4.
Laboratory tests: Standard-of-care comprehensive metabolic panel, complete blood counts, and coagulation study results will be recorded. Each participating site will process its own standard-of-care laboratory tests. Interventions will be determined by the attending physicians and not influenced by the study personnel.
-
5.
Urinary studies: Standard-of-care urinalysis, urine microscopy, cytology, and urine chemistry study results will be recorded.
After the development of AKI, the following information will be collected:
-
1.
Renal replacement therapies: The reason for initiating dialysis, type of dialysis, start and stop dates of dialysis, and dialysis discontinuation will be recorded.
-
2.
Survival status: The subject’s survival status will be established by review of the EMR and telephone contact with patient at hospital discharge and days 28 and 90.
-
3.
Serum creatinine: Standard-of-care laboratory assessment of Scr will be recorded from the EMR at hospital discharge and days 28 and 90.
Adjudication
Cases must pass adjudication by a panel of adult and pediatric nephrologists to be included the final analysis. Kidney injury is often multifactorial, and adjudication is required to determine the underlying causes.13 An adjudication process for evaluating DIKD has not been previously developed. Prior published processes for adjudication of drug-induced liver injury and skin hypersensitivity were used as a framework for designing adjudication of DIKD.14, 15 Two independent nephrologists will review each blinded case, presented as a summary of completed data (Supplementary Figure S1), to ascertain causality for DIKD and to assess the contribution of risk factors for the development of kidney injury. Specifically, the adjudicator will make a determination of DIKD, and will evaluate the relative contribution of each causal drug and the relative contribution of recorded AKI risk factors (Supplementary Appendix S1). Considering that adjudication processes for DIKD have not been previously published, this study will investigate the reliability and validity of this adjudication tool.
Biological Samples
Each subject will provide 15 to 50 ml of urine for biomarkers and 15 ml of blood for DNA and biomarkers. DNA will be isolated from a whole-blood sample and stored at the UCSD O’Brien Core Laboratory for genetic analysis. Centers will retain a 5-ml blood sample for DNA at their site for back-up if the original sample sent to the O’Brien Center was lost or destroyed.
DNA Preparation and Genotyping
Genomic DNA will be prepared from blood leukocytes at UCSD’s Institute for Genomic Medicine facility, and samples will be genotyped at the Broad Institute, using the Illumina Human Core plus Exome (or similar GWAS) array.
Protocol Definitions
Because DIKD includes different mechanisms and sites of injury, the clinical presentation can be categorized into 4 major phenotypes, including AKI, tubular dysfunction, glomerular disorders, and nephrolithiasis (Table 2).
AKI
A process that causes an abrupt reduction in kidney function and is defined by meeting any of the following criteria16:
-
(i)
An absolute increase in Scr (≥0.3 mg/dl or ≥26.4 μmol/l) (within 48-hour time window) from the reference Scr value.
-
(ii)
A percentage increase in Scr of ≥50% (1.5-fold from reference) within 7 days.
-
(iii)
A reduction in urine output (documented oliguria of <0.5 ml/kg/h for >6 hours) despite adequate fluid resuscitation when applicable.
-
(iv)
An absolute decrease in Scr of ≥0.3 mg/dl or ≥26.4 μmol/l (within 48-hour time window) from the reference Scr.
-
(v)
A relative decrease in Scr of ≥50% (1.5-fold from reference) within 7 days.
Subacute DIKD
A process that causes a slower reduction in kidney function and is defined by meeting any of the following criteria:
-
(i)
A percentage increase in Scr of ≥50% (1.5-fold from reference) occurring between 7 and 90 days after the initiation of the drug or within 2 weeks of drug discontinuation.
-
(ii)
A relative decrease in Scr of ≥50% (1.5-fold from reference) within 90 days of a change in drug dosing or discontinuation.
CKD
Prior evidence of markers of kidney damage for ≥3 months (microalbuminuria or proteinuria or abnormalities in imaging tests) or the presence of glomerular filtration rate (GFR) < 60 ml/min/1.73 m2 for ≥3 months calculated with the Modification of Diet in Renal Disease (MDRD) equation.17, 18 Chronic kidney disease will be staged from stage 1 to 5 based on the calculated CKD-EPI or Ckid (pediatrics) GFR.19, 20
Reference Scr to Determine Timing of AKI
The following criteria will be used in order of preference, depending on available values:
-
a)Lowest Scr immediately prior to index event. Must meet the following criteria:
-
a.Precedes drug exposure
-
b.Within 90 days of index event
-
c.Closest value to index event
-
d.Lowest value prior to drug exposure
-
e.If no Scr within 90 days of index event, will use the hospital admission creatinine value
-
a.
-
b)
For declining Scr criteria with no prior reference level, will use the lowest value post drug reduction or stoppage as reference
-
c)For AKI phenotype, will have 2 reference Scr values:
-
a.Reference 1:
-
i.Lowest value within 90 days of initiation of primary drug
-
i.
-
b.Reference 2:
-
i.Lowest value closest to initiation of drug
-
i.
-
a.
Baseline Scr to Determine CKD Status
Creatinine values >90 days from index event
-
(i)
Lowest values within 90 days to 12 months to establish eGFR stage based on CKD-EPI or Ckid (pediatrics)
-
(ii)
Historical evidence of CKD based on standard criteria: proteinuria, biopsy, ultrasound findings
-
(iii)
Imaging studies consistent with CKD
-
(iv)
For chronic drug exposure, need values prior to drug initiation
New-Onset AKI
Evidence of AKI without prior evidence of kidney damage and calculated MDRD GFR ≥ 90 mL/min/1.73 m2.
AKI-on-CKD
Evidence of AKI with criteria for kidney damage as defined above, occurring in a patient with CKD criteria, will be considered as AKI-on-CKD.
Definition of End of Trial
The end of trial is the date of the last follow-up of the last patient.
Withdrawal of Patients/Subjects
Patients may withdraw or may be withdrawn from the study for any of the following reasons:
-
•
Patient decides not to continue with the study
-
•
Administrative decision by the investigator
-
•
Significant protocol deviation
-
•
Patient is unable to provide adequate blood sample for DNA
-
•
Case does not pass adjudication by adjudication committee
Assessment of Safety
Because this is an observational cohort study, the main risks include that of blood sampling and loss of confidentiality. Measures will be taken to minimize those risks. All adverse and serious adverse events will be reported to the appropriate ethics committees.
Genetic Data Management and Quality Control
Data management of the large amount of genotype data and quality control (QC) will be performed using the software PLINK.21 Initial data cleaning will include multi-step standard procedures.22 In short, QC steps include removal of samples and single nucleotide polymorphisms (SNPs) with low genotyping quality, genetic assessment of sex and ancestry to flag inconsistencies with self-report, and assessment of cryptic relatedness of subjects, and will result in a filtered dataset of high quality.
Procedure for Accounting of Missing Data
To maximize information present in our data and to allow for a potential comparison of our results across multiple studies genotyped on other platforms, we will impute genotypes of SNPs not present on our array. Imputation will be performed using IMPUTE2, a method found to be especially useful in the context of samples including mixed ancestries.23 Reference data will include phased haplotypes from the 1000 Genomes Project.24
Based on the distribution of our study sites, we anticipate the inclusion of several major ethnic/racial groups (Europeans/European Americans, African Americans, Asians, and Hispanics) as well as admixed individuals from different ancestries. It is well established that allele frequencies across the genome can vary among individuals of different ancestral groups, and allelic association studies including subjects of different biogeographic ancestry are at high risk for this artifact. In addition, locus heterogeneity can lead to false-negative results due to variation in genetic backgrounds. We will take advantage of the large amount of genotypic information available and will control for potential population stratification in a 2-step process.25 First, we will identify major groups of subjects with similar biogeographic ancestry, using approaches such as the program STRUCTURE.26 The inclusion of population reference samples compiled by our group27 will increase the power of these approaches. Analyses are then conducted separately on these more homogeneous ancestry groups, including principal components (PCs) derived from the program EIGENSOFT v3.028 to control for additional population stratification.
Statistical Analysis
In this observational cohort study, descriptive statistics will be calculated for demographic and baseline characteristics including the following: demographics; baseline characteristics; past medical history; initial health status measures; composition of sample and patient location (e.g., surgical vs. medical ICU); concurrent care (e.g., medications, interventions including surgery, and invasive procedures); drug exposure (dose, frequency, route, timeline); renal function estimates (Scr, GFR).
Genomewide Association Study
We will perform a GWAS to examine the association of genetic variants with the risk for development of DIKD. Mapping genetic determinants of DIKD requires a multi-level approach, including an understanding of interactions between environmental stressors (i.e., AKI risk factors) and individual constitutional factors.
Logistic regression models will be used to test for associations between SNPs and case/control status under the assumption of an additive genetic model. Initially, we will adjust each phenotype for the typical covariates of age, sex, indices of ancestry, and study cohort. Additional covariates predicted to be of high importance include AKI risk factors such as volume status, concomitant nephrotoxins, comorbidities such as diabetes or hypertension, and so forth. To assess significance thresholds and to correct for multiple comparisons, we will use conventional methods such as Bonferroni correction for a genome-wide approach (i.e., P < 5 × 10−8), permutation tests to derive an empirical level of significance, and false-discovery rate analysis. PLINK and R code will be used to conduct these analyses.
The highly polymorphic HLA system has been shown to be especially important in adverse drug reactions. In order to derive classical HLA alleles, we will take advantage of the dense SNP coverage in the major histocompatibility complex (MHC) region of the Illumina Human Core plus Exome array. Specific methods as developed by Zheng et al. take advantage of the extended haplotype structure within the MHC to reliably predict HLA alleles based on genotypes from these arrays.29
Sample Size
A total sample of approximately 800 patients will be enrolled via 40 clinical centers worldwide. In previous studies examining the genetic predisposition to drug-induced liver injury (DILI) and serious skin reactions, the associations between certain polymorphisms in HLA and aforementioned injuries were highly significant.5, 6 In a study of DILI caused by flucloxacillin, possession of the HLA-B5701* allele was associated with an odds ratio (OR) of 80.6 (95% confidence interval [CI], 22.8−284.9) for this adverse effect.5 The HLA-A*3101 allele, with a prevalence of 2% to 5% in Northern Europeans, was found to be a risk factor for carbamazepine-induced hypersensitivity syndrome (OR, 12.41; 95% CI, 1.27−121.03), maculopapular exanthema (OR, 8.33; 95% CI, 3.59−19.36), and Stevens Johnson−toxic epidermal necrosis (OR, 25.93; 95% CI, 4.93−116.18).6
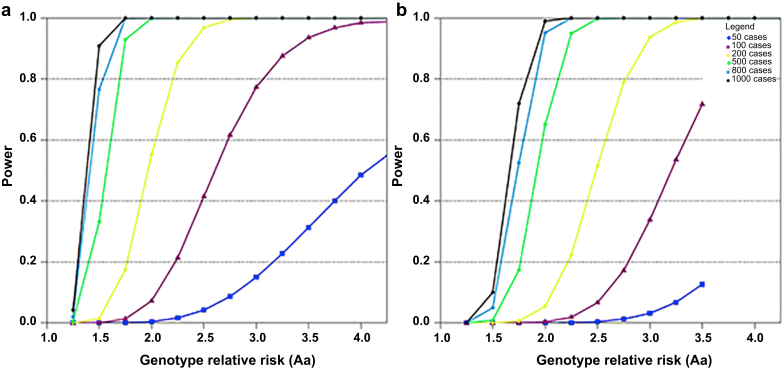
We conducted power calculations for our proposed case-control association study based on a range of realistic assumptions for trait heritability: assuming an additive model, type I error rate of 5 × 10−8 for the GWAS, perfect linkage disequilibrium between marker and trait allele for common alleles (minor allele frequency [MAF] 5%−20%) and a disease prevalence of 20%. We conservatively base our calculations below on n = 6,000 POPRES controls, which are treated as unselected population controls.30 However, we note that at the time of analysis we will take advantage of additional available control subjects, which will increase the power of the study. Figure 2 shows the number of subjects (cases plus controls) required to achieve 80% power to detect a locus with a specific genotype relative risk (GRR), considering a range of number of cases and 6000 controls, respectively, for a MAF of 20% (Figure 2a) and a MAF of 5% (Figure 2b), which is typical for the HLA alleles. These calculations are considering a joint analysis of all subjects together, subsets of cases within a particular phenotype (N = 50−600) as well as medication-specific analyses within a drug class or specific drug (N = 50−600). Assuming an incidence of 20% of an adverse reaction to a specific medication, in a sample of N = 600 cases and 6000 population controls, we would have 80% power to detect a locus with a small GRR of 1.6 in the case of a common SNP with MAF = 20% (panel A). Power is reduced for rare alleles, and we will have power to detect a locus with a GRR of 1.98 in the case of a rare allele with a MAF = 5% (Figure 2b).
Figure 2.
Sample size estimation for the Genetic Contribution to Drug Induced Renal Injury (DIRECT) study. This figure demonstrates the power estimates for individual drugs, drug classes, and phenotypes for common alleles. (a) minor allele frequency = 20%, (b) minor allele frequency = 5%.
Discussion
The DIRECT study is the first cohort study designed to evaluate whether there is a genetic basis for drug-induced nephrotoxicity. Other networks have been developed in DILI and serious skin injury, but large networks for DIKD have not been previously established. Many genetic studies on DIKD to date have focused on single drugs or classes of drugs. The DIRECT study is the first study to establish an international network of centers enrolling cases of DIKD from a broad range of drugs. Data from analysis of the genome will provide preliminary information on significant genetic polymorphisms associated with DIKD from each drug. This will enhance our understanding of mechanisms of toxicity for each drug. The DIRECT study will provide preliminary information on target genes to further validate the utility of genetic screening in addition to current clinical testing for prediction of risk.
Strengths of the DIRECT study include the broad enrollment of patients of different ages from various clinical scenarios, standardization of the DIKD phenotype, detailed information on the course of injury and clinical risk factors, and the causality assessment process.
With the inclusion of hospitalized and ambulatory care patients with DIKD from various countries, the DIRECT study will inform on the spectrum of DIKD, variation in drug use and practice patterns in the different age groups and countries. We anticipate variation in co-morbidities and causal drugs in pediatric compared to adult patients. In addition, drug use and practice patterns vary internationally. We anticipate that genetic susceptibility will vary by race and ethnicity. The DIRECT study will capture a global snapshot from countries in North and South Americas, Europe, and Asia.
The development of standardized phenotypes for DIKD was critical to studying genetic susceptibility. All cases must meet standard criteria for enrollment. Using inclusion criteria of stage 2 AKI will improve the specificity of cases and enhance causality, thereby increasing the likelihood of finding genetic susceptibility. Moreover, phenotype standardization will assist clinicians, researchers, industry, and regulatory bodies in designing future studies of DIKD.
The design of this study captures real-life clinical scenarios including complex patients with multiple co-morbidities, risk factors, and multi-drug injury. Acute kidney injury is multi-factorial and requires assessment of the contribution of competing risk factors to injury. Detailed information on comorbidities, concurrent risk factors, and the timing and extent of drug exposures will enable the complete description of the spectrum of injury in DIKD, risk factors for DIKD by causal drug, and outcomes of injury.
The development of an adjudication process for nephrotoxicity is novel and will provide insight into causality assessment and attribution of risk. Clinical adjudication has been previously used in AKI biomarker studies, in which adjudicators are presented with information on a patient’s Scr values and asked to make a judgement as to whether AKI was present or absent.31 However, adjudication of DIKD requires additional consideration of all potential contributing factors to AKI. Adjudication is a complex process requiring evaluation of causality using published criteria, including the following: strength of association; temporality; consistency of the adverse event in different subjects; specificity of the drug for the adverse event; biological gradient for the effect (dose−response relationship); plausibility; coherence; experimental evidence that can alter the adverse event; and analogy between drugs of the same class.32 The gold standard for adjudication is expert consensus. Two independent nephrologists will adjudicate each case, with a third acting as a tiebreaker. Published causality assessment tools will be used and compared to one another for DIKD. These tools can help reduce disagreement between adjudicators and classify the relationship likelihood. A limitation of these general causality assessment tools is lack of assessment of competing risk factors. Acute kidney injury is a syndrome with multiple etiologies and many contributing risk factors. Assessment of these risk factors improves the specificity of cases and ultimately strengthens causal association. However, the method for attributing risk to each risk factor has not been previously delineated, which may lead to variability in these assessments. Adjudicator intra- and interrater reliability will inform on the effectiveness of tools for causality assessment. We developed case report forms for adjudication, which were pilot tested by 2 adjudicators and refined for clarity (Supplementary Appendix S1). We created a Web-based adjudication platform where adjudicators could access their randomly assigned cases. All adjudicators were trained to complete the adjudication electronically in a blinded process. The data from adjudication will capture the complexity of causality assessment in DIKD. The adjudication pass rates for each of the causal drugs in DIKD will inform on the complexity of cases and the consideration for sample size determination in the design of future studies.
Limitations of this study include the lack of drug-matched controls for GWAS as well as the lack of validated causality assessment tools for nephrotoxicity. We acknowledge that drug-matched controls would enhance the ability to use drug exposure data in clinical risk profiling. However, obtaining such controls requires time and resources, and, given the low incidence and the severity of nephrotoxicity, we opted to use population-based controls, as this has been successfully done in other studies of serious, rare adverse events.5, 6, 10 In addition, the OR ranged from 8.33 to 80.6 for DILI and serious skin injury, which suggests that the DIRECT study cohort size is adequate to detect DIKD-associated polymorphisms with OR > 2. Validated causality assessment tools in DIKD are lacking. The Naranjo et al.33 and Liverpool tools14 have not been validated in DIKD but do perform well for determining causality of general adverse drug events. By using these tools in addition to the adjudication process, we hope to refine the causality assessment of DIKD leading to the development of validated tools.
Information obtained from causality assessment, attribution of risk from concurrent risk factors, together with genetic determinants of injury may be used to develop predictive risk scores for DIKD. The results of the DIRECT study may translate into safer patient outcomes through individualization of therapy based on the patient’s clinical risk factors and genotype.
Disclosure
The authors have received funding from the International Serious Adverse Events Consortium to conduct this study. Trial Registration: NCT02159209 at www.ClinicalTrials.gov.
Footnotes
Supplementary Appendix S1. DIRECT study case report forms.
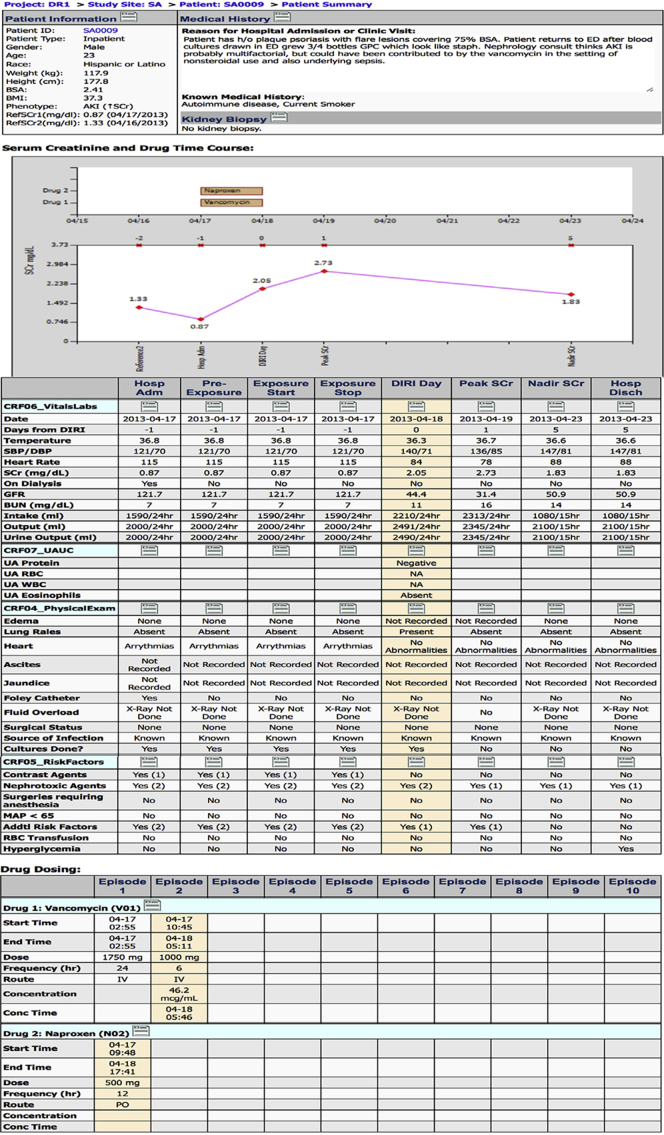
Figure S1. Patient summary report. This figure captures the patient summary report that is presented to adjudicators at the time of adjudication. The data are extracted from various case report forms and presented in a visual format to summarize the patient's medical history, time course for drug exposure, trend in serum creatinine (Scr), vital signs, concurrent acute kidney injury (AKI) risk factors at each time point, and drug-dosing history.
Supplementary material is linked to the online version of the paper at http://www.kireports.org.
Supplementary Material
DIRECT study case report forms.
Figure S1.
Patient summary report. This figure captures the patient summary report that is presented to adjudicators at the time of adjudication. The data are extracted from various case report forms and presented in a visual format to summarize the patient’s medical history, time course for drug exposure, trend in serum creatinine (Scr), vital signs, concurrent acute kidney injury (AKI) risk factors at each time point, and drug-dosing history.
References
- 1.Hagos Y., Wolff N.A. Assessment of the role of renal organic anion transporters in drug-induced nephrotoxicity. Toxins (Basel) 2010;2:2055–2082. doi: 10.3390/toxins2082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filipski K.K., Mathijssen R.H., Mikkelsen T.S. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joh K., Aizawa S., Yamaguchi Y. Drug-induced hypersensitivity nephritis: lymphocyte stimulation testing and renal biopsy in 10 cases. Am J Nephrol. 1990;10:222–230. doi: 10.1159/000168085. [DOI] [PubMed] [Google Scholar]
- 4.Spanou Z., Keller M., Britschgi M. Involvement of drug-specific T cells in acute drug-induced interstitial nephritis. J Am Soc Nephrol. 2006;17:2919–2927. doi: 10.1681/ASN.2006050418. [DOI] [PubMed] [Google Scholar]
- 5.Daly A.K., Donaldson P.T., Bhatnagar P. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 6.McCormack M., Alfirevic A., Bourgeois S. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364:1134–1143. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Link E., Parish S., Armitage J. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 8.Mehta R.L., Awdishu L., Davenport A. Phenotype standardization for drug-induced kidney disease. Kidney Int. 2015;88:226–234. doi: 10.1038/ki.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association Standards of Medical Care in Diabetes—2016 Abridged for Primary Care Providers. Clin Diabetes. 2016;34:3–21. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly A.K., Day C.P. Genetic association studies in drug-induced liver injury. Semin Liver Dis. 2009;29:400–411. doi: 10.1055/s-0029-1240009. [DOI] [PubMed] [Google Scholar]
- 11.Pannu N., James M., Hemmelgarn B., Klarenbach S., Alberta Kidney Disease Network Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8:194–202. doi: 10.2215/CJN.06480612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawhney S., Mitchell M., Marks A. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open. 2015;5:e006497. doi: 10.1136/bmjopen-2014-006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta R.L., Pascual M.T., Soroko S. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher R.M., Kirkham J.J., Mason J.R. Development and inter-rater reliability of the Liverpool Adverse Drug Reaction Causality Assessment Tool. PLoS One. 2011;6:e28096. doi: 10.1371/journal.pone.0028096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maria V.A., Victorino R.M. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997;26:664–669. doi: 10.1002/hep.510260319. [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 17.Levey A.S., Coresh J., Greene T. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 18.Levey A.S., Bosch J.P., Lewis J.B. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz G.J., Munoz A., Schneider M.F. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purcell S., Neale B., Todd-Brown K. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson C.A., Pettersson F.H., Clarke G.M. Data quality control in genetic case-control association studies. Nat Protocols. 2010;5:1564–1573. doi: 10.1038/nprot.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howie B., Marchini J., Stephens M. Genotype imputation with thousands of genomes. G3. 2011;1:457–470. doi: 10.1534/g3.111.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siva N. 1000 Genomes project. Nat Biotechnol. 2008;26:256. doi: 10.1038/nbt0308-256b. [DOI] [PubMed] [Google Scholar]
- 25.Smith E.N., Bloss C.S., Badner J.A. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009:14755–14763. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falush D., Stephens M., Pritchard J.K. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nievergelt C.M., Maihofer A.X., Shekhtman T. Inference of human continental origin and admixture proportions using a highly discriminative ancestry informative 41-SNP panel. Investig Genet. 2013;4:13. doi: 10.1186/2041-2223-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price A.L., Patterson N.J., Plenge R.M. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 29.Zheng X., Shen J., Cox C. HIBAG-HLA genotype imputation with attribute bagging. Pharmacogenom J. 2014;14:192–200. doi: 10.1038/tpj.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson M.R., Bryc K., King K.S. The Population Reference Sample, POPRES: a resource for population, disease, and pharmacological genetics research. Am J Hum Genet. 2008;83:347–358. doi: 10.1016/j.ajhg.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bihorac A., Chawla L.S., Shaw A.D. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189:932–939. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 32.Hill A.B. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 33.Naranjo C.A., Busto U., Sellers E.M. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DIRECT study case report forms.