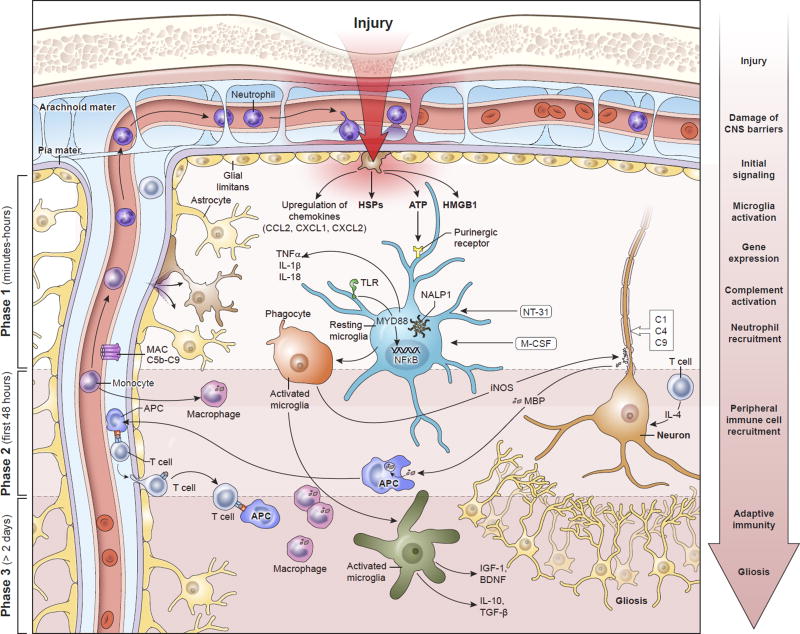
Figure 1. Temporal progression of the immune response to contusion TBI.
Phase I begins within minutes of brain injury due to the release of alarmins from the damaged meninges, glial limitans, and parenchyma, such as ATP, HSPs, HGMB1, etc. These signals bind to PAMP and DAMP sensors like TLRs and purinergic receptors that induce immediate activation of resident myeloid cells (e.g. microglia) and inflammasome assembly (NALP1) that promotes the generation of mature IL-1β and IL-18. In addition, NFκB translocates to the nuclei of these cells and induces an immunological program involving cellular proliferation and the release inflammatory amplifiers such as chemokines, cytokines, ROS, and NO, among others. Phase 1 also includes complement activation and the recruitment of neutrophils to the meninges and perivascular spaces. Neutrophil recruitment depends in part on purinergic receptor signaling. Secondary damage to CNS tissue occurs in Phase 1 and can continue into Phase 2. This can be mediated by inflammatory cytokines, complement, and ROS. T cells and monocytes are recruited to the damage site in Phase 2, where monocytes convert into macrophages and T cells have the ability to produce neuroprotective cytokines in response to alarmins. Macrophages participate in the cleanup of debris and damaged cells. Based on their state of functional activation, they can either promote further damage or initiate the process of inflammatory resolution and tissue repair. Inflammation can continue for an extended period of time into Phase 3. Self-antigens released from damaged neural cells can be presented by local APCs to T cells. The ideal outcome during Phase 3 is resolution of the inflammatory response, release of trophic factors, and isolation damaged areas via astrocytes. However, this is does not always occur following TBI and chronic inflammation can persist. Abbreviations: APC, antigen-presenting cell; ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; CCL2, chemokine (C-C motif) ligand 2; CXCL1, chemokine (C-X-C motif) ligand 1; CXCL2, chemokine (C-X-C motif) ligand 2; DAMP, damage associated molecular pattern molecules; HSPs, heat shock proteins; HMGB1, high mobility group box 1 protein; IGF-1, insulin-like growth factor-1; IL-1, interleukin-1; IL-4, interleukin-4; IL-10, interleukin-10; iNOS, inducible nitric oxide synthase; MyD88, myeloid differentiation primary response gene 88; NALP1, NAcht leucine-rich repeat protein 1; NT-3, neurotrophin-3; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; MBP, myelin basic protein; M-CSF, macrophage colony stimulating factor; NO, nitric oxide; PAMP, pathogen-associated molecular pattern; ROS, reactive oxygen species; TGF-β, transforming growth factor beta; TLR, toll-like receptors; TNF-α, tumor necrosis factor-alpha