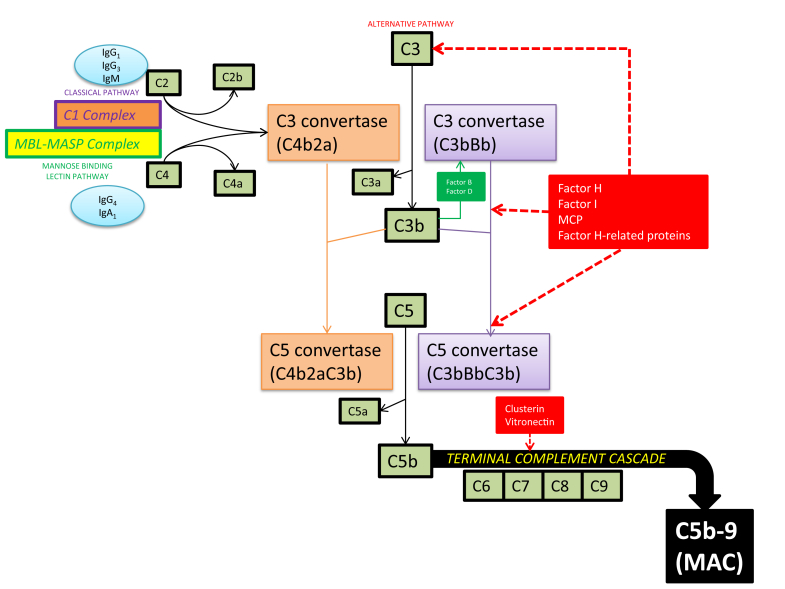
Figure 1.
An overview of the complement cascade. The classical, mannose-binding lectin and alternative complement pathways converge at C3 to generate an enzyme complex known as C3 convertase that cleaves C3 into C3a and C3b. However, the pathways are distinct in their points of origin. The classical complement pathway is activated by either IgG (predominantly IgG1 and IgG3) or IgM antibodies bound to antigen; this immune complex exposes a binding site on the Ig for the first component of the classical pathway, C1, to form a C1 complex that cleaves C2 and C4. The lectin pathway is initiated by the binding of mannose-binding lectin (MBL) to the polysaccharide surface of pathogenic bacteria. This binding results in the formation of a trimolecular complex with mannose-binding lectin-associated serine proteases (MASPs) and subsequent cleavage of C2 and C4. IgG4 and IgA1 autoantibodies can also bind MBL and activate the lectin complement pathway. The alternative pathway begins at the level of C3 and is constitutively active via spontaneous hydrolysis of C3 to C3b, which binds factor B to yield the C3 convertase (C3bBb) of this pathway. Hence, activation of this complement pathway is generally considered antibody independent. MAC, membrane attack complex; MCP, membrane cofactor protein.