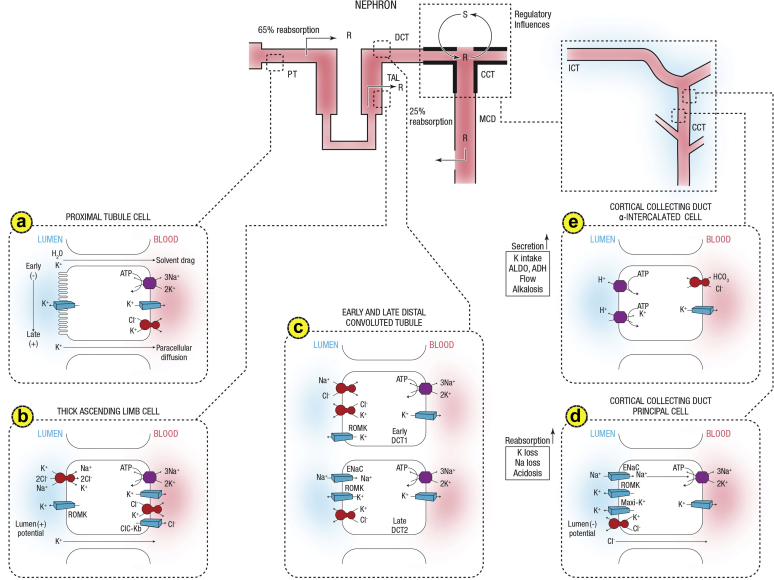
Figure 1.
Summary of potassium transport along the nephron. Following filtration, potassium is extensively reabsorbed along the proximal tubule and the loop of Henle. Potassium is secreted along the initial and cortical collecting tubules. Net secretion can be replaced by net reabsorption in states of potassium depletion. Also shown are the 2 cell types lining the distal tubule and cortical collecting duct. (a) A cell model for K+ transport in the proximal tubule. K+ reabsorption in the proximal tubule primarily occurs through the paracellular pathway. Active Na+ reabsorption drives net fluid reabsorption across the proximal tubule, which, in turn, drives K+ reabsorption through a solvent drag mechanism. As fluid flows down the proximal tubule, the luminal voltage shifts from slightly negative to slightly positive. The shift in transepithelial voltage provides an additional driving force favoring K+ diffusion through the low-resistance paracellular pathway. Experimental studies suggest that there may be a small component of transcellular K+ transport; however, the significance of this pathway is not known. K+ uptake through the Na+-K+-ATPase pump can exit the basolateral membrane through a conductive pathway or coupled to Cl–. An apically located K+ channel functions to stabilize the cell negative potential, particularly in the setting of Na+-coupled cotransport of glucose and amino acids, which has a depolarizing effect on cell voltage. (b) A cell model for K+ transport in the thick ascending limb of Henle. K+ reabsorption occurs by both paracellular and transcellular mechanisms. The basolateral Na+-K+-ATPase pump maintains intracellular Na+ at a low level, thus providing a favorable gradient to drive the apically located Na+-K+-2Cl– cotransporter (an example of secondary active transport). The apically located renal outer medullary K+ (ROMK) channel provides a pathway for K+ to recycle from cell to lumen, and ensures an adequate supply of K+ to sustain Na+-K+-2Cl– cotransport. This movement through ROMK creates a lumen-positive voltage, providing a driving force for passive K+ reabsorption through the paracellular pathway. Some of the K+ entering the cell through the cotransporter exits the cell across the basolateral membrane, accounting for transcellular K+ reabsorption. K+ can exit the cell through a conductive pathway or in cotransport with Cl–. ClC-Kb is the primary pathway for Cl– efflux across the basolateral membrane. (c) A cell model for K+ transport in the distal convoluted tubule (DCT). In the early DCT, luminal Na+ uptake is mediated by the apically located thiazide-sensitive Na+-Cl– cotransporter. The transporter is energized by the basolateral Na+-K+-ATPase, which maintains low intracellular Na+ concentration, thus providing a favorable gradient for Na+ entry into the cell through secondary active transport. The cotransporter is abundantly expressed in the DCT1 but progressively declines along the DCT2. ROMK is expressed throughout the DCT and into the cortical collecting duct. Expression of the epithelial Na+ channel (ENaC), which mediates amiloride-sensitive Na+ absorption, begins in the DCT2 and is robustly expressed throughout the downstream connecting tubule and cortical collecting duct. The DCT2 is the beginning of the aldosterone-sensitive distal nephron as identified by the presence of both the mineralocorticoid receptor and the enzyme 11β-hydroxysteroid dehydrogenase II. This enzyme maintains the mineralocorticoid receptor free to only bind aldosterone by metabolizing cortisol to cortisone, which has no affinity for the receptor. Electrogenic-mediated K+ transport begins in the DCT2 with the combined presence of ROMK, ENaC, and aldosterone sensitivity. Electroneutral K+-Cl– cotransport is present in the DCT and collecting duct. Conditions that promote a low luminal Cl– concentration increase K+ secretion through this mechanism, which occurs with delivery of poorly reabsorbable anions, such as sulfate, phosphate, or bicarbonate. (d) The cell that is responsible for K+ secretion in the initial collecting duct and the cortical collecting duct is the principal cell. This cell possesses a basolateral Na+-K+-ATPase that is responsible for the active transport of K+ from the blood into the cell. The resultant high cell K+ concentration provides a favorable diffusion gradient for movement of K+ from the cell into the lumen. In addition to establishing a high intracellular K+ concentration, the activity of this pump lowers intracellular Na+ concentration, thereby maintaining a favorable diffusion gradient for movement of Na+ from the lumen into the cell. The movements of both Na+ and K+ across the apical membrane occur through well-defined Na+ and K+ channels. (e) Reabsorption of HCO3 in the distal nephron is mediated by apical H+ secretion by the α-intercalated cell. Two transporters secrete H+, a vacuolar H+-ATPase and an H+-K+-ATPase. The H+-K-ATPase uses the energy derived from ATP hydrolysis to secrete H+ into the lumen and reabsorb K+ in an electroneutral fashion. The activity of the H+-K+-ATPase increases in K+ depletion and thus provides a mechanism by which K+ depletion enhances both collecting duct H+ secretion and K+ absorption.33 ADH, antidiuretic hormone; ALDO, aldosterone; ASDN, aldosterone-sensitive distal nephron; ATPase, adenosine triphosphatase; CCT, cortical collecting tubule; Cl, chloride; ClC-Kb, chloride channel Kb; DCT, distal convoluted tubule; ENaC, epithelial sodium channel; HCO3, bicarbonate; ICT, initial connecting tubule; MCD, medullary collecting duct; PT, proximal tubule; R, reabsorption; ROMK, renal outer medullary potassium; S, secretion; TAL, thick ascending limb. Courtesy of Murray Epstein.