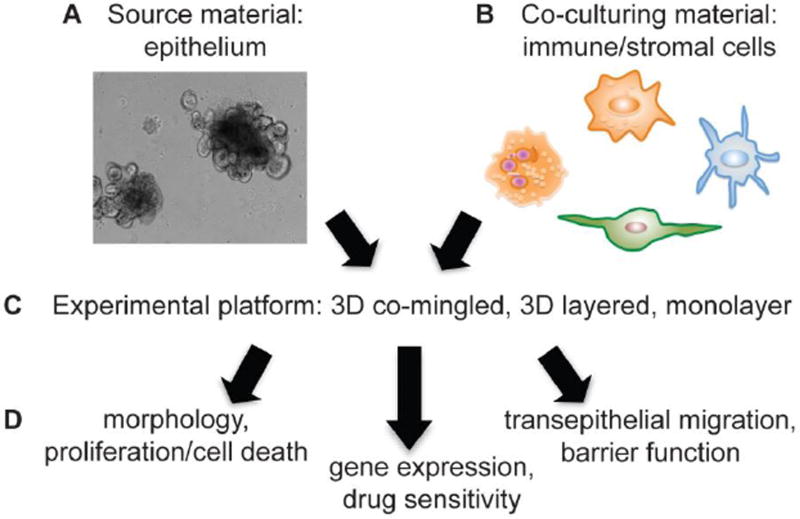
Figure 1.
Steps to utilize 3D enteroids/colonoids/tumoroids for co-culture experiments. A. Enteroid/colonoid/tumoroid cultures can be grown readily from normal crypt (stem-cell containing) epithelium or tumors from mouse models or patient tissue. B. Studies demonstrating co-cultures of enteroid/colonoid/tumoroid with various inflammatory components are rapidly emerging. Considerations for co-culturing include: which immune/stromal cell co-culture experiment will answer my experimental question? Can this immune/stromal cell type be readily purified from mice/humans for use in co-culture experiments? Will the co-cultured cells survive in 3D enteroid growth conditions? If possible, will the co-culture cells express a fluorescent reporter? C. Co-culture platform must be considered and can include co-mingling of enteroid cells with immune/stromal cells (permitting direct interaction), growing adherent cells (such as fibroblasts) and then layering enteroids in 3D matrix on top of the layer of adherent cells, or evaluating dissociated enteroids grown as monolayers on semi-permeable supports with co-culture cells added to the upper (apical) or lower (basolateral) chamber. D. Analyses of co-cultures can include using biochemical or immunohistochemical analyses of morphology, proliferation, and cell death, gene expression studies, drug sensitivity, and functional tests including transepithelial migration and barrier function.