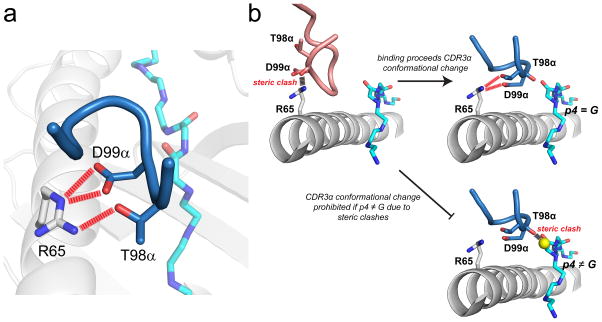
Figure 1.
High peptide specificity emerging from how a TCR interfaces with the MHC protein. A) In the structure of the A6 TCR bound to Tax11-19/HLA-A2, Thr98 and Asp99 of CDR3α form strong electrostatic interactions with Ar65 on the HLA-A2 α1 helix (29). B) In order to interact with Arg65, CDR3α must undergo a conformational change upon binding (31). In the absence of the loop conformational change, steric clashes would occur between the CDR3α backbone and Arg65. Upon making the conformational change, the backbone of CDR3α is tightly packed against position 4 of the peptide. If position 4 is anything other than glycine, steric clashes would exist, preventing the loop from adopting its needed conformation, as shown in bottom right for an alanine at position 4.